Cheat Sheet: Chemical Reactions and Equations | Science Class 10 PDF Download
| Table of contents |

|
| Chemical Reactions |

|
| Chemical Equations |

|
| Types of Chemical Reactions |

|
| Effects of Oxidation in Everyday Life |

|
| Key Activities and Observations |

|
Chemical Reactions
A process where substances (reactants) undergo a chemical change to form new substances (products) is called chemical reaction.
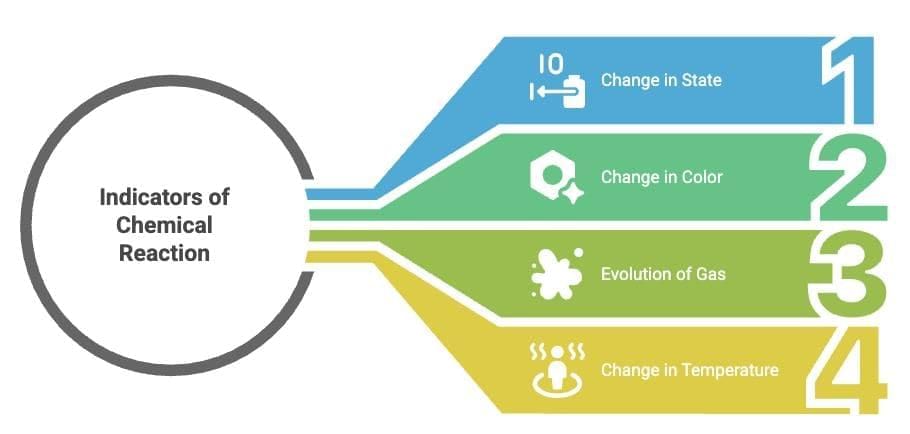
Indicators of chemical reaction:
Change in state
Change in color
Evolution of a gas
Change in temperature
Examples:
Milk turning sour (fermentation)
Rusting of iron
Cooking of food
Digestion and respiration
Chemical Equations
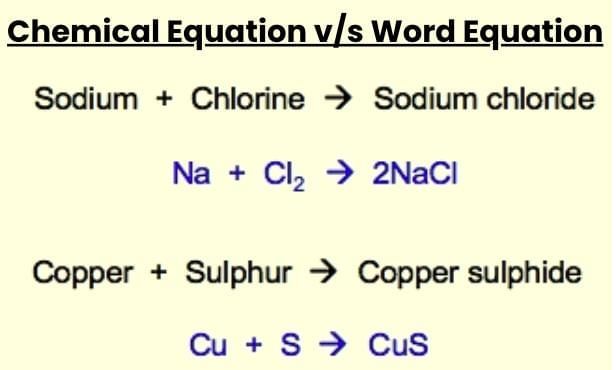
Word Equation: Describes reactants and products in words, e.g., Magnesium + Oxygen → Magnesium oxide.
Chemical Equation: Uses chemical formulae, e.g., Mg + O₂ → MgO (skeletal, unbalanced).
Balanced Chemical Equation: Ensures the number of atoms of each element is equal on both sides, following the Law of Conservation of Mass.
Example: 2Mg + O₂ → 2MgOBalancing Steps (Hit-and-Trial Method):
Write the skeletal equation.
List the number of atoms for each element on both sides.
Balance the element with the maximum atoms first (e.g., in Fe + H₂O → Fe₃O₄ + H₂, start with Fe₃O₄).
Use coefficients to equalize atoms (e.g., 3Fe + 4H₂O → Fe₃O₄ + 4H₂).
Verify all atoms are balanced.
Add physical states: (s) for solid, (l) for liquid, (g) for gas, (aq) for aqueous.

Balanced Chemical Equation with States: 3Fe(s) + 4H₂O(g) → Fe₃O₄(s) + 4H₂(g)
Types of Chemical Reactions
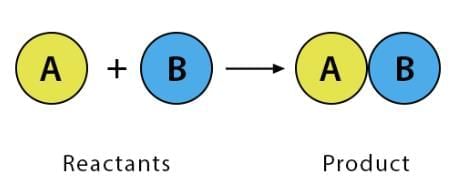
1. Combination Reaction
Two or more reactants combine to form a single product.
Characteristics: Often exothermic (releases heat).
Examples:
CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat (Quick lime + Water → Slaked lime)
C(s) + O₂(g) → CO₂(g) (Burning of coal)
2H₂(g) + O₂(g) → 2H₂O(l) (Formation of water)
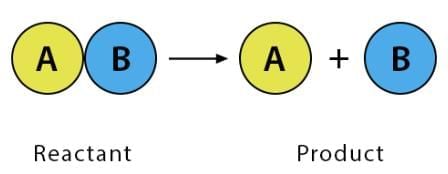
2. Decomposition Reaction
A single reactant breaks down into two or more products.
Characteristics: Often endothermic (requires energy: heat, light, or electricity).
Examples:
Thermal Decomposition: CaCO₃(s) → CaO(s) + CO₂(g) (Heat)
Photolytic Decomposition: 2AgCl(s) → 2Ag(s) + Cl₂(g) (Sunlight)
Electrolytic Decomposition: 2H₂O(l) → 2H₂(g) + O₂(g) (Electricity)
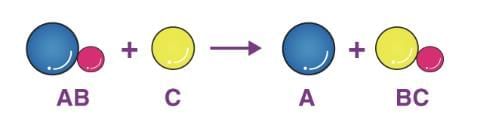
3. Displacement Reaction
A more reactive element displaces a less reactive element from its compound.
Example: Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s) (Iron displaces copper)
Other Examples:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Pb(s) + CuCl₂(aq) → PbCl₂(aq) + Cu(s)
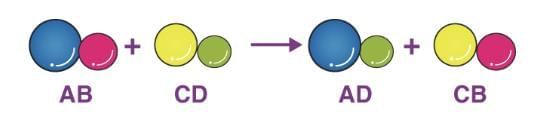
4. Double Displacement Reaction
Exchange of ions between two reactants, often forming a precipitate.
Example: Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq) (Precipitation reaction)
Characteristics: Produces an insoluble product (precipitate).
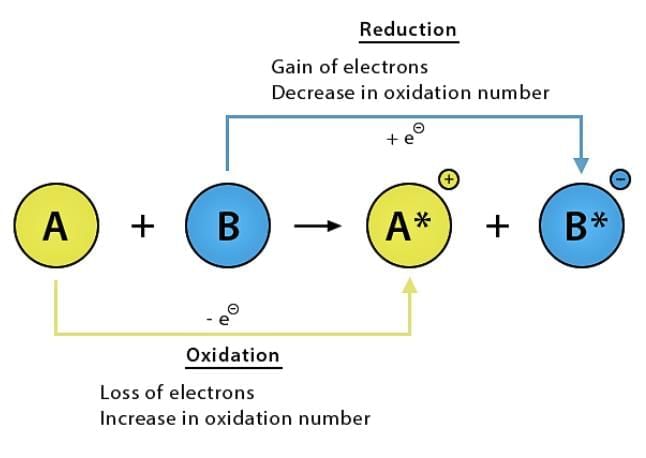
5. Oxidation and Reduction (Redox Reactions)
Oxidation: Gain of oxygen or loss of hydrogen.
Example: 2Cu + O₂ → 2CuO (Copper oxidized to copper oxide)
Example: 4Na(s) + O₂(g) → 2Na₂O(s) (Sodium oxidized)
Reduction: Loss of oxygen or gain of hydrogen.
Example: CuO + H₂ → Cu + H₂O (Copper oxide reduced to copper)
Example: ZnO + C → Zn + CO (Zinc oxide reduced to zinc)
Redox Reaction: Involves both oxidation and reduction.
Example: MnO₂ + 4HCl → MnCl₂ + 2H₂O + Cl₂ (HCl oxidized, MnO₂ reduced)
Effects of Oxidation in Everyday Life
Corrosion
When metals are attacked by substances like moisture or acids, forming oxides or other compounds its called corrosion.
Examples:
Rusting of iron: Fe forms reddish-brown Fe₂O₃·nH₂O.
Green coating on copper: Cu forms CuCO₃·Cu(OH)₂.
Black coating on silver: Ag forms Ag₂S.
Impact: Damages iron structures (e.g., bridges, ships). Prevented by painting or galvanizing.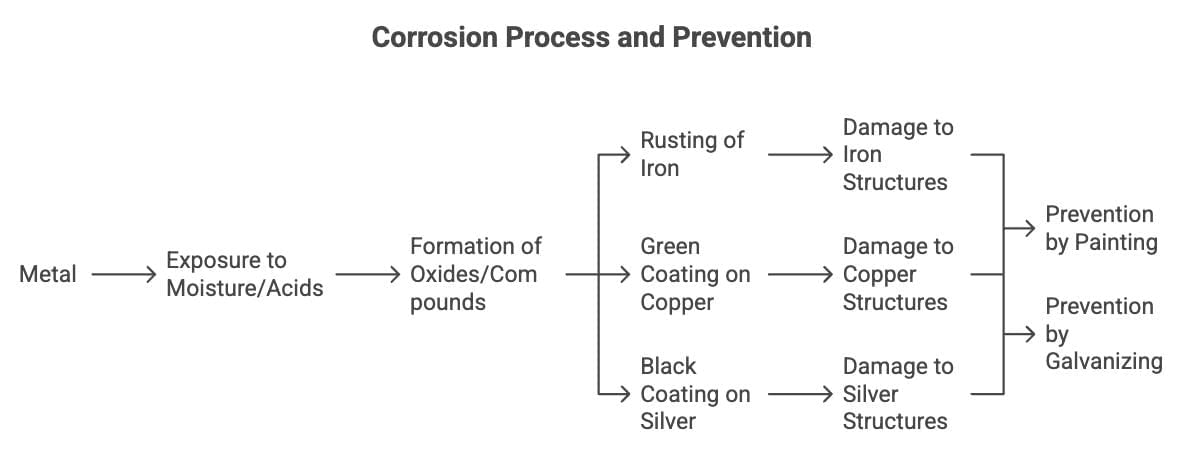
Rancidity
Rancidity is oxidation of fats/oils in food, causing bad smell/taste.
Prevention
- Use antioxidants
- Store in air-tight containers flushed with nitrogen (e.g., in chip bags).
Key Activities and Observations
Activity (Magnesium Burning): Mg burns with a dazzling white flame, forms white MgO powder (exothermic, combination).
Activity (Zinc + Acid): Zn + H₂SO₄ → ZnSO₄ + H₂ (gas evolution, temperature increase).
Activity (CaO + Water): Exothermic, forms Ca(OH)₂, used in whitewashing.
Activity (Ferrous Sulphate): Green FeSO₄·7H₂O decomposes to brown Fe₂O₃, SO₂, SO₃ (thermal decomposition).
Activity (Lead Nitrate): Decomposes to PbO, NO₂ (brown fumes), O₂ (thermal decomposition).
Activity (Electrolysis of Water): Produces H₂ (double volume) and O₂.
Activity (Silver Chloride): White AgCl turns grey Ag in sunlight (photolytic decomposition).
Activity (Iron + CuSO₄): Ironystyrene nail turns brownish, blue CuSO₄ fades (displacement).
Activity (Na₂SO₄ + BaCl₂): Forms white BaSO₄ precipitate (double displacement).
Activity (Copper Oxidation): Cu turns black CuO on heating (oxidation).
|
82 videos|677 docs|80 tests
|
FAQs on Cheat Sheet: Chemical Reactions and Equations - Science Class 10
| 1. What is a chemical reaction and how is it represented in a chemical equation? |  |
| 2. What are the different types of chemical reactions? |  |
| 3. How does oxidation affect our everyday life? |  |
| 4. What observations can be made during chemical reactions? |  |
| 5. Why is balancing chemical equations important? |  |