Class 10 Science Chapter 1 Question Answers - Chemical Reactions and Equations
Q1: What happens when magnesium ribbon burns in the air?
Ans: When magnesium ribbon burns in the air, it combines with the oxygen to form magnesium oxide.
2Mg(s) + O2 (g) → 2MgO(s)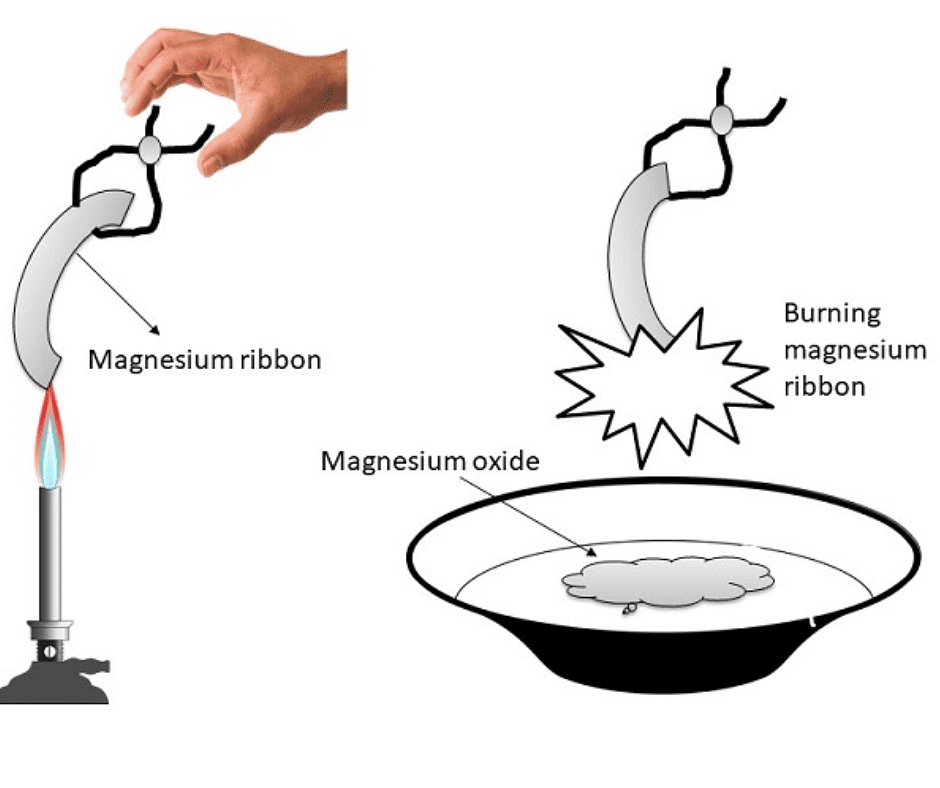
Q2: On what chemical law, the balancing of chemical equations is based?
Ans: The balancing of a chemical equation is based on the law of conservation of mass.
Q3: Name the gas that evolved when zinc reacts with dil. HCl.
Ans: Hydrogen gas is evolved.
When HCl , which is an acid goes into the chemical reaction with the Zinc metal, the reaction occurs by vigorously bubbles, which are due to the formation of Hydrogen gas.
Q4: Name and state the law which is kept in mind while we balance a chemical equation.
Ans: Law of conservation of mass. Mass can neither be created nor destroyed during a chemical reaction.
Q5: Write the chemical equation for reactions that take place when lead nitrate and potassium iodide solutions are mixed.
Ans: Pb(NO3)2 + 2KI → 2KNO3 + PbI2
Lead nitrate + Potassium iodide → Potassium nitrate + Leadiodide
Q6: Why is photosynthesis considered an endothermic reaction?
Ans: Photosynthesis is considered an endothermic reaction because heat is absorbed in this process.
Q7: What is precipitate?
Ans: Precipitate is an insoluble metal compound formed after a reaction.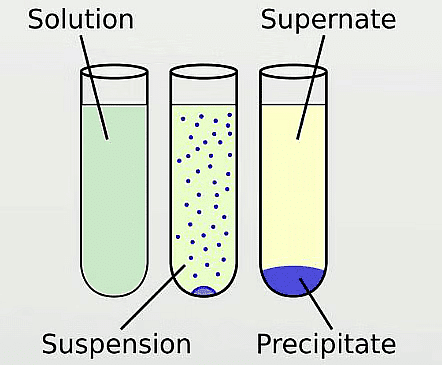
Q8: Why is the combustion of Liquified Petroleum Gas (LPG) a chemical change?
Ans: Combustion of Liquified Petroleum Gas (LPG) is a chemical change because, after its combustion, a new substance is formed and cannot be turned back into LPG.
Q9: What is wrong with the following equation?
Mg + O ➝ MgO
Identify the mistake and balance the equation.
Ans: In this equation, oxygen should be in molecular form (O2).
2Mg + O2 ➝ 2MgO.
Q10: What is meant by the skeletal equation?
Ans: The equation where the number of atoms of each element on both sides of a chemical equation is not equal is called a skeletal equation.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science Chapter 1 Question Answers - Chemical Reactions and Equations
| 1. What is a chemical reaction? |  |
| 2. What is a chemical equation? |  |
| 3. How can we balance a chemical equation? |  |
| 4. What are the types of chemical reactions? |  |
| 5. What is the importance of chemical reactions in everyday life? |  |






















