Class 10 Science Chapter 2 Question Answers - Acids, Bases and Salts
Q1: What should be done as remedy if stung by leaves of nettle plant in the wild?
Ans: The area should be rubbed with the leaf of dock plant. Dock PlantQ2: What happens when nitric acid is added to egg shell?
Dock PlantQ2: What happens when nitric acid is added to egg shell?
Ans: Eggshell is made of calcium carbonate. When nitric acid is added to the shell calcium nitrate, carbon dioxide and water are formed.
CaCO3 + 2HNO3 → Ca(NO3)2 + CO2 + H2O
Q3: What is the concentration of H+ ion in pure water?
Ans: 10-7
Q4: Which one of these has a higher concentration of H+ ions? 1 M HCl or 1 M CH3COOH.
Ans: 1 M HCl has higher concentration of H+ ions.
Q5: Name an example of olfactory indicators.
Ans: There are compounds known as olfactory indicators that smell differently in acidic and basic solutions. By using a procedure called olfactory titration, these types of indicators are employed in laboratories to determine whether a given solution is basic or acidic.
Vanilla extract and onion are examples. Olfactory IndicatorsQ6: Name the chemical substance present in thick white and yellowish clouds present in the atmosphere of Venus.
Olfactory IndicatorsQ6: Name the chemical substance present in thick white and yellowish clouds present in the atmosphere of Venus.
Ans: Sulphuric acid.
Q7: What is acid rain?
Ans: Rainwater having pH less than 5.6, is called acid rain.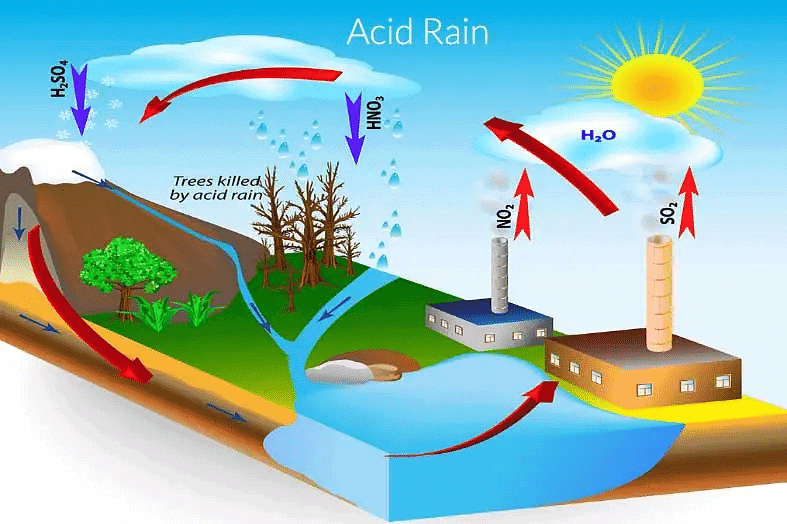 Q8: Name the hardest substance in the body.
Q8: Name the hardest substance in the body.
Ans: Tooth enamel (Calcium phosphate).
Q9: The pH of three solutions A, B and C are 4, 9 and 6 respectively. Arrange them in increasing order of acidic strength.
Ans: The increasing order of acidic strength is : B < C < A.
Q10: Name the chemist who had given the pH scale.
Ans: S.P.L. Sorensen (1909).
Q11: Name the acid present in tomato.
Ans: Oxalic acid.
Q12: Acidic and basic solutions in water conduct electricity. Why?
Ans: Because they produce hydrogen and hydroxide ions respectively.
Q13: What would be the colour of litmus in a solution of sodium carbonate?
Ans: Red litmus will change to blue in sodium carbonate solution.
Q14: The pH of a sample of vegetable soup was found to be 6.5. How is this soup likely to taste?
Ans: The taste will be slightly sour as it is weakly acidic.
Q15: Name the chemical substance which is used in the manufacture of soap as well as used as a preservative in pickles.
Ans: Sodium chloride (NaCl).
Q16: There are two jars A and B containing food materials. Food in jar ‘A’ is pickled with acetic acid while ‘B’ is not. Food of which of jar will stale first? Explain. Name two synthetic indicators which are used to test acids and bases.
Ans: Food in jar ‘B’ will stale first because it will undergo oxidation and will also be attacked by microorganisms.
Synthetic indicators: Phenolphthalein, methyl orange.
Q17: What is the chemical formula of soda ash?
Ans: Na2CO3
Soda Ash is the common name for sodium carbonate, which is a chemical compound.
Q18: Name the substance used for disinfecting drinking water supply.
Ans:
Bleaching powder is calcium oxychloride or calcium hypochlorite with chemical formula CaOCl2
. It is marketed as chlorine powder for water treatment and as bleaching agent. It is used to sanitize pools and disinfect drinking water.
 Q19: Name a chemical substance which can be used to detect the presence of moisture in a liquid.
Q19: Name a chemical substance which can be used to detect the presence of moisture in a liquid.
Ans: Anhydrous copper sulphate.
Q20: What is meant by water of crystallisation?
Ans: Water of crystallisation is the fixed number of water molecules chemically attached to each formula unit of a salt in its crystalline form.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science Chapter 2 Question Answers - Acids, Bases and Salts
| 1. What is an acid? |  |
| 2. What is a base? |  |
| 3. What are salts? |  |
| 4. How do acids and bases react with each other? |  |
| 5. What are the uses of acids, bases, and salts? |  |

















