Daniell Cell & Galvanic Cell | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Daniell cell |

|
| Galvanic Cells |

|
| Some Important Terms |

|
| Solved Example |

|
Daniell cell
It is designed to make use of the spontaneous redox reaction between zinc and cupric ions to produce an electric current. It consists of two half-cells. The half-cells on the left contain a zinc metal electrode dipped in ZnSO4 solution.
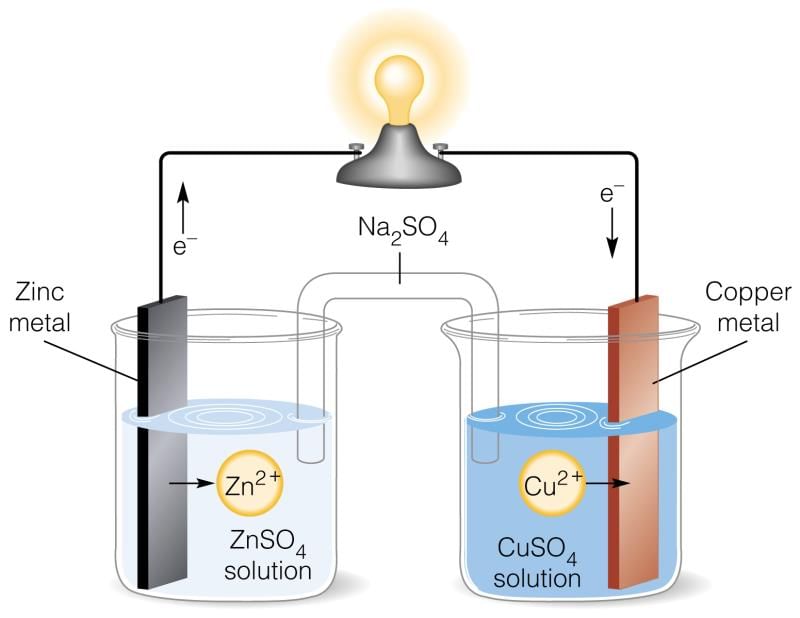 Daniell cell
Daniell cell
The half-cell on the right consists of the copper metal electrode in a solution CuSO4. The half-cells are joined by a salt bridge that prevents the mechanical mixing of the solution.
When the zinc and copper electrodes are joined by a wire, the following observations are made:
- There is a flow of electric current through the external circuit.
- The zinc rod loses its mass while the copper rod gains in mass.
- The concentration of ZnSO4 solution increases while the concentration of copper sulphate solution decreases.
- The solutions in both the compartments remain electrically neutral.
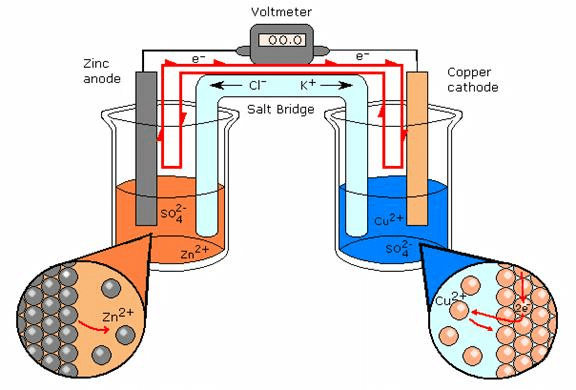
During the passage, if the electric current through the external circuit, electrons flow from the zinc electrode to the copper electrode. At the zinc electrode, the zinc metal is oxidized to zinc ions which go into the solution.
The electrons released at the electrode travel through the external circuit to the copper electrode where they are used in the reduction of Cu2+ ions to metallic copper which is deposited on the electrode. Thus, the overall redox reaction is:
Zn(s) + Cu2+ → Cu(s) + Zn2++(aq)
Thus, indirect redox reaction leads to the production of electrical energy. At the zinc rod, oxidation occurs. It is the anode of the cell and is negatively charged while at the copper electrode, reduction takes place; it is the cathode of the cell and is positively charged.
Thus, the above points can be summed up as:
- Voltaic or Galvanic cell consists of two half-cells. The reactions occurring in half-cells are called half-cell reactions. The half-cell in which oxidation taking place is called oxidation half-cell and the reaction taking place in it is called oxidation half-cell reaction. Similarly, the half-cell that occurs is called reduction half-cell and the reaction taking place in it is called reduction half-cell reaction.
- The electrode where oxidation occurs is called the anode and the electrode where reduction occurs is termed cathode.
- Electrons flow from anode to cathode in the external circuit.
- Chemical energy is converted into electrical energy.
- The net reaction is the sum of two half-cell reactions. The reaction is Daniell cell can be represented a
| Oxidation half reaction | Reduction half reaction | Net reaction |
Zn(s) → Zn2+(aq) + 2e- | Cu2+(aq) + 2e- → Cu (s) | Zn(s) + Cu2+ (aq) → Zn2+(aq) + Cu(s) |
Electrode Signs
The signs of the anode and cathode in the voltaic or galvanic cells are opposite to those in the electrolytic cells.
Electrolytic Cell | Volatic or Galvanic Cell |
E.m.f. is applied to cell | E.m.f. is generated by cell |
Anode Cathode | Voltaic or Galvanic cell Anode Cathode | |
Sign Electron flow Half-reaction | + - Out in Oxidation reduction | - + Out in Oxidation reduction |
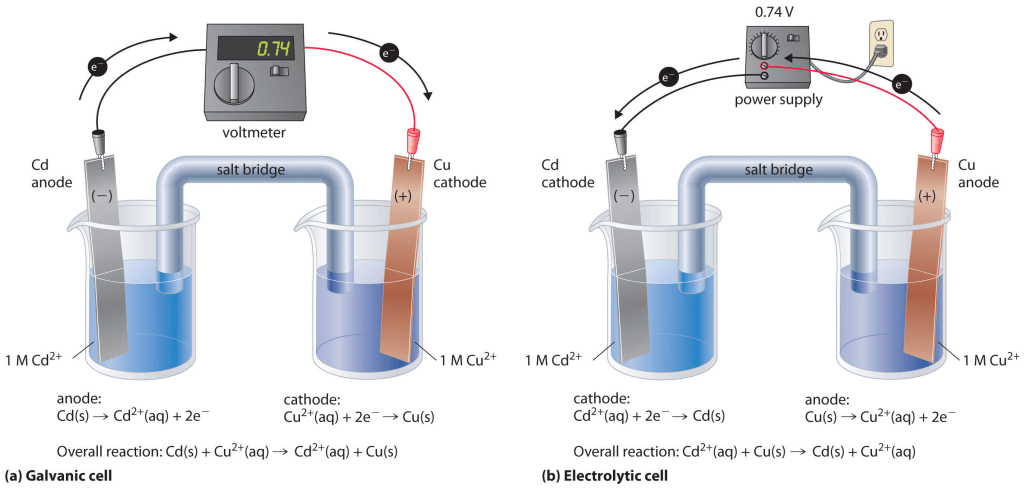
Reversible and Irreversible Cells
Daniell cell has the emf value 1.09 volt. If an opposing emf exactly equal to 1.09 volt is applied to the cell, the cell reaction,
Zn + Cu2+→ Cu + Zn2+
stops but if it is increased infinitesimally beyond 1.09 volt, the cell reaction is reversed.
Cu + Zn2+→ Zn + Cu2+
Such a cell is termed a reversible cell. Thus, the following are the two main conditions of reversibility:
- The chemical reaction of the cell stops when an exactly equal opposing emf is applied.
- The chemical reaction of the cell is reversed and the current flows in the opposite direction when the opposing emf is slightly greater than that of the cell.
- Any other cell which does not obey the above two conditions is termed irreversible.
- A cell consisting of zinc and copper electrodes dipped into the solution of sulphuric acid is irreversible.
- Similarly, the cell Zn|H2S04(aq)|Ag is also irreversible because when the external emf is greater than the emf of the cell, the cell reaction,
- Zn + 2H+ → Zn2+ + H2 is not reversed but the cell reaction becomes 2Ag + 2H+ → 2Ag+ + H2
Galvanic Cells
Among other cells, a galvanic cell is a type of electrochemical cell. It is used to supply electric current by making the transfer of electrons through a redox reaction. A galvanic cell is an exemplary idea of how energy can be harnessed using simple reactions between a few given elements. It is amazing to study how a galvanic cell can be set up and utilized to obtain energy.
Explaining in the most simple terms, a galvanic cell acts as a device in which simultaneous oxidation and reduction reactions take place. These reactions are used to convert chemical energy into electrical energy, which can be utilized for any commercial purpose.
Working of Galvanic Cells
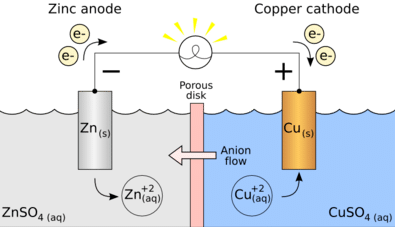
The galvanic cell utilizes the ability to separate the flow of electrons in the process of oxidization and reduction, causing a half-reaction and connecting each with a wire so that a path can be formed for the flow of electrons through such wire.
This flow of electrons is essentially called a current. Such current can be made to flow through a wire to complete a circuit and obtain its output in any device such as a television or a watch.
A galvanic cell can be made out of any two metals. These two metals can form the anode and the cathode if left in contact with each other. This combination allows the galvanic corrosion of that metal which is more anodic. A connecting circuit shall be required to allow this corrosion to take place.
Setup of a Galvanic Cell
In order to create a galvanic cell, one would have to go through the following setup. The cell would ideally include two electrodes. One of these electrodes, the cathode, shall be a positively charged electrode while the other, shall be the anode, the negatively charged electrode.
These two electrodes shall form the two essential components of the galvanic cell. The chemical reaction related to reduction shall take place at the cathode while the oxidation half-reaction shall take place at the anode. As has already been said, any two metals can be used to create a chemical reaction.Understanding the Galvanic Cell with an Example
Let us take an example where the two metals involved in the chemical reaction are zinc and copper. As the chemical reaction takes place, Zinc would end up losing two electrons. This will be taken up by copper to become elemental copper.
Since these two metals will be placed in two separate containers and would be connected by a conducting wire, an electric current would be formed, which would transfer all electrons from one metal to another.At the same time, the two metals shall be immersed in a salt solution, say, Zinc sulphate and Copper sulphate in this case. In this case, the two solutions are not mixed together directly but can be joined using a bridge or a medium.
This medium shall be responsible for the transfer of ions but also make sure that the two solutions do not come to mix with each other.
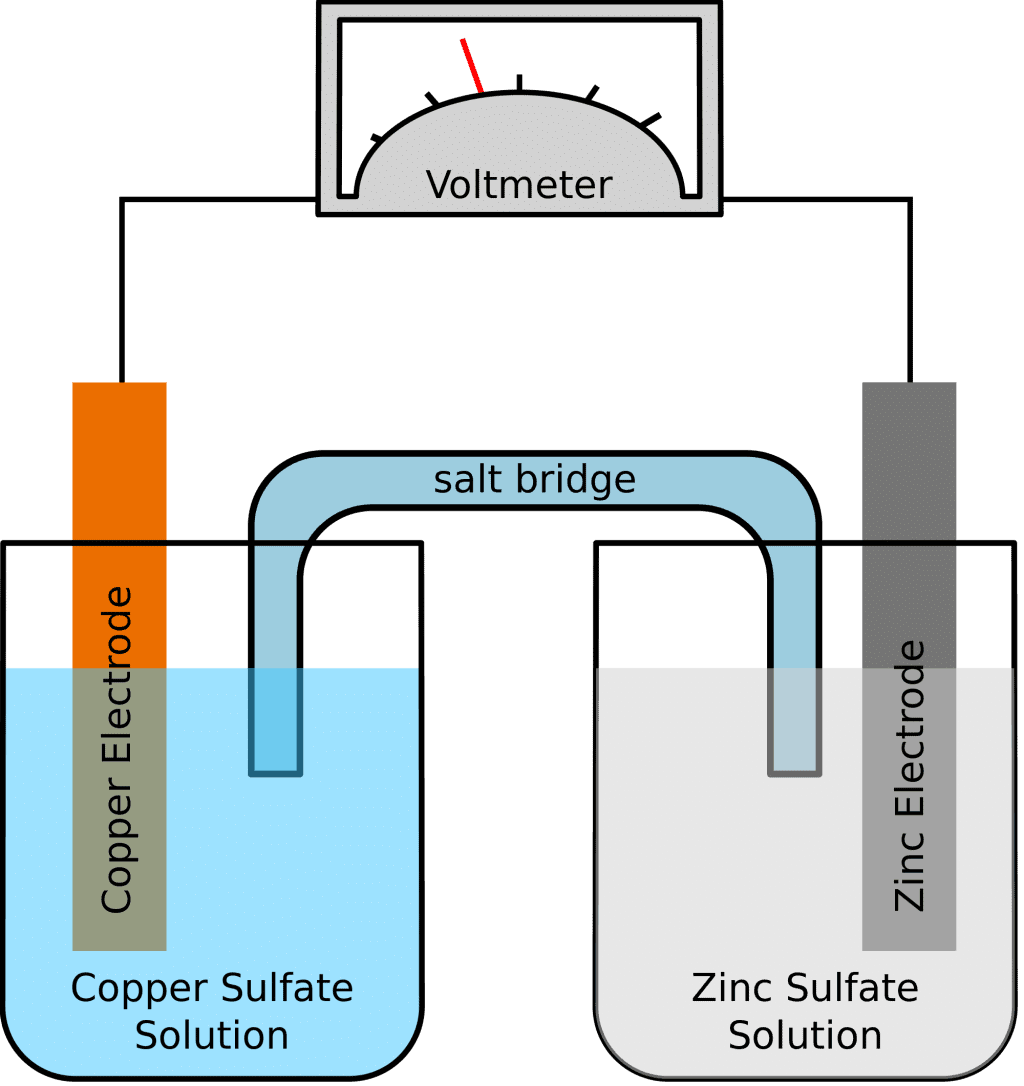
Such a bridge helps in completing the circuit for carrying the electric charge and also makes sure that the solutions in the containers with the metals remain neutral and do not mix with each other.
As long as the salt bridge does not interfere with the redox reaction, under which oxidization and reduction are taking place, it does not matter which salt bridge is being used in the chemical reaction.
Some Important Terms
Some of the important terms brought into use in galvanic cells are listed below:
Phase boundaries: It refers to the two metals which act as cathode and anode.Salt bridge: The connecting bridge or medium that allows a redox reaction to take place.
Oxidation and reduction: The chemical processes that allow the electric current to form and flow through a galvanic cell.
Solved Example
Q1. In a galvanic cell, what would happen if no salt bridge is used while the redox reaction takes place?
Ans: In the absence of a salt bridge within each container containing the metals, the redox reaction would begin in much the same way. But in the absence of the salt bridge, the same would come to an end rather abruptly. The respective solutions will not be able to maintain their electric neutrality. Other than this, there will be no change in the chemical reaction or any alteration of any type because of the absence of the salt bridge or medium.
|
75 videos|278 docs|78 tests
|
FAQs on Daniell Cell & Galvanic Cell - Chemistry Class 12 - NEET
| 1. What is a Daniell cell? |  |
| 2. How does a galvanic cell work? |  |
| 3. What are the important terms associated with galvanic cells? |  |
| 4. How does a Daniell cell generate electricity? |  |
| 5. What are some applications of galvanic cells? |  |





















