Diagram Based Questions: Metals and Non-metals | Science Class 10 PDF Download
Q1: Answer the following questions based on the diagram given below:
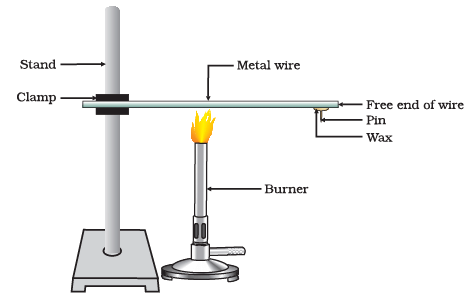 (i) What is the purpose of clamping the metal wire on a stand in the experiment?
(i) What is the purpose of clamping the metal wire on a stand in the experiment?
Ans: The wire is clamped on a stand to hold it in place while heating and to ensure that it doesn't move during the experiment.
(ii) What is the role of the pin fixed to the free end of the wire using wax?
Ans: The pin allows us to observe any changes in the wire's behavior as it is heated, specifically to see if it melts or undergoes any other noticeable changes.
(iii) Which metals are known as the best conductors of heat?
Ans: The best conductors of heat are silver and copper.
(iv) What does the experiment demonstrate about the conductivity of heat in metals?
Ans: The experiment demonstrates that metals are good conductors of heat because the metal wire heats up when exposed to a heat source.
(v) Which metals are comparatively poor conductors of heat?
Ans: Lead and mercury are mentioned as comparatively poor conductors of heat.
Q2: Answer the following questions based on the diagram given below:
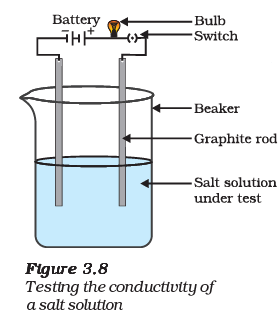
(i) What is the purpose of the experiment depicted in the diagram?
Ans: The purpose of the experiment is to test the conductivity of a salt solution.
(ii) Describe the setup shown in the diagram used to test the conductivity of the salt solution.
Ans: The setup includes a battery or power source, wires, two electrodes (usually made of metal), and a container with the salt solution. The electrodes are connected to the battery, forming a closed circuit through the solution.
(iii) Explain why the bulb in the circuit lights up when the salt solution is added.
Ans: The bulb lights up because the salt solution allows the flow of electric current. This happens because salt (an ionic compound) dissociates into ions in the solution, allowing the flow of charged particles (ions) between the electrodes, completing the electrical circuit and lighting up the bulb.
(iv) What would happen if you replaced the salt solution with distilled water in this experiment?
Ans: If distilled water is used instead of a salt solution, the bulb would not light up. Distilled water does not contain ions in significant quantities, so it does not conduct electricity as effectively as a salt solution.
(v) What is the significance of this experiment in real-life applications?
Ans: This experiment helps us understand the concept of electrical conductivity and is important in various real-life applications. For example, it is used in testing the purity of water, determining the strength of electrolyte solutions in batteries, and in various industries where the conductivity of solutions is crucial for processes such as metal plating and chemical manufacturing.
Q3: Answer the following questions based on the diagram given below:
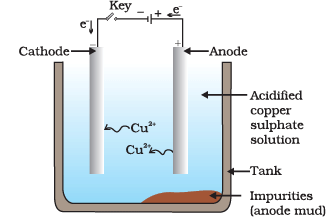
(i) What is the purpose of the experiment shown in the image?
Ans: The purpose of this experiment is to purify impure copper using the process of electrolytic refining.
(ii) What is the role of the anode in this experiment?
Ans: The anode is made of impure copper and it serves as the source of copper ions during the electrolysis process.
(iii) Why is the cathode made of pure copper?
Ans: The cathode is made of pure copper to allow the deposition of pure copper ions from the electrolyte onto the cathode, thus purifying the copper.
(iv) What is the composition of the electrolyte used in this experiment?
Ans: The electrolyte used in this experiment is a solution of acidified copper sulfate.
(v) What happens when an electric current is passed through the setup shown in the image?
Ans: When an electric current is passed through the setup, copper ions from the impure anode dissolve into the electrolyte, move towards the cathode, and get deposited as pure copper on the cathode, leaving impurities behind in the anode.
Q4: Answer the following questions based on the diagram given below:
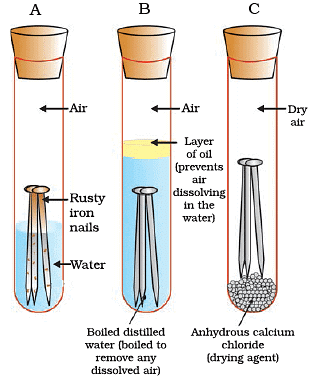
(i) What is the purpose of this experiment?
Ans: The purpose of this experiment is to study the conditions under which iron rusts and observe how the presence or absence of air and water affects the rusting of iron.
(ii) What are the three different tubes labeled in the experiment, and what do they represent?
Ans: The three tubes are labeled A, B, and C. Tube A represents the condition where both air and water are present, Tube B represents the condition with no air dissolved in the water, and Tube C represents the condition where the air is dry.
(iii) Why is it essential to have water present in Tube A for this experiment?
Ans: Water is essential in Tube A because rusting of iron typically involves the reaction of iron with oxygen and water vapor from the air. So, to study rusting, both air and water are required.
(iv) What is the role of Tube B in this experiment, and how does it differ from Tube A?
Ans: Tube B helps us understand the role of air in the rusting process. It differs from Tube A as it has water but no dissolved air, which allows us to observe if the presence of air is necessary for rusting.
(v) What is the significance of Tube C in the experiment, and what does it tell us about the rusting process?
Ans: Tube C is important as it contains dry air. It helps us determine whether moisture (water vapor) in the air is necessary for iron to rust. If rusting occurs in Tube C, it suggests that moisture in the air is not required for rusting.
|
80 videos|569 docs|80 tests
|
FAQs on Diagram Based Questions: Metals and Non-metals - Science Class 10
| 1. What are some common properties of metals? |  |
| 2. How do metals and non-metals differ in terms of reactivity? |  |
| 3. Can you provide examples of common metals and non-metals? |  |
| 4. Why are metals generally good conductors of electricity? |  |
| 5. How do the physical properties of metals and non-metals influence their uses in everyday life? |  |

















