UPSC Exam > UPSC Notes > Geography for UPSC CSE > Earth's Atmosphere: Composition & Structure
Earth's Atmosphere: Composition & Structure | Geography for UPSC CSE PDF Download
| Table of contents |

|
| What is Atmosphere? |

|
| Importance of Atmosphere |

|
| Composition of the Atmosphere |

|
| Structure of the Atmosphere |

|
| Based on Composition |

|
| Based on Change in Temperature: |

|
What is Atmosphere?
The envelope of gases surrounding the earth is called the atmosphere. It forms a protective boundary between outer space and the biosphere.
- It is a mixture of odorless, colorless, tasteless, and formless gases mixed and blended so thoroughly that it acts as a single gas.
- The present atmosphere's gases are not the direct residue of the early stage of the earth’s formation.
- They are a product of progress through volcanic eruptions, hot springs, chemical breakdowns of solid matter, and redistribution from the biosphere.
Importance of Atmosphere
- The atmosphere is a significant component of the biospheric ecosystem because life on the earth’s surface is because of this atmosphere; otherwise, the earth would have become barren like the moon.
- The atmosphere contains living gases like oxygen for man and animal and carbon dioxide for plants(necessary for survival).
- It protects the earth from the harmful radiation from the sun. It acts as a greenhouse by allowing short-wave radiation (from sun) and trapping long-wave terrestrial radiation (from Earth’s surface).
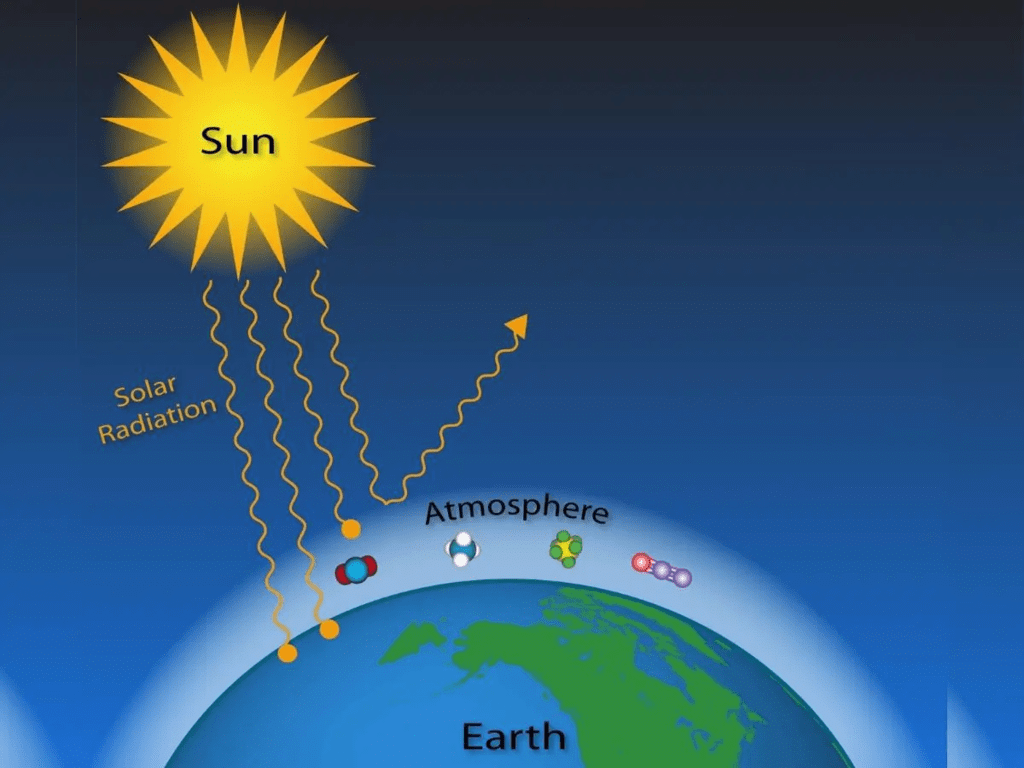
- All life forms need a particular range of temperature and a specific range of solar radiation frequencies to carry out their biophysical processes. The atmosphere absorbs certain frequencies and lets through some other frequencies of solar radiation. In other words, the atmosphere regulates the entry of solar radiation.
- The atmosphere also keeps the temperature over the earth’s surface within certain limits. In the absence of the atmosphere extremes of temperature would exist between day and night over the earth’s surface.
- The atmosphere also takes care of extra-terrestrial objects like meteors that get burnt up while passing through the atmosphere (mesosphere to be precise) due to friction.
Question for Earth's Atmosphere: Composition & Structure
Try yourself:Atmosphere is a boundary between
View Solution
Composition of the Atmosphere
The atmosphere is composed of –
- Gases
- Vapour
- Particulates
The atmosphere is a mixture of many gases. Also, it contains enormous numbers of solid and liquid particles, collectively called aerosols.
1. Gases
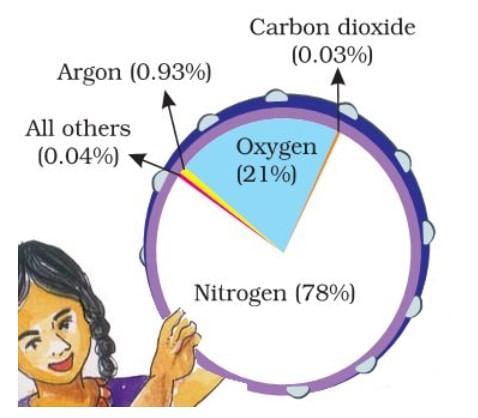
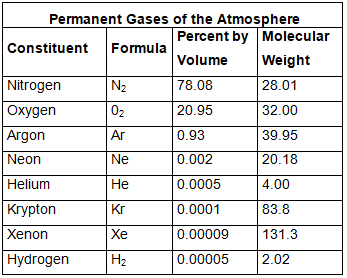
- Nitrogen and oxygen make up nearly 99% of the clean, dry air. The remaining gases are mostly inert and constitute about 1% of the atmosphere.
- Although constituting only 21% of the atmosphere's total volume, Oxygen is the most crucial component among gases. All living organisms inhale oxygen. Besides, oxygen can combine with other elements to form essential compounds, such as oxides. Also, combustion is not possible without oxygen.
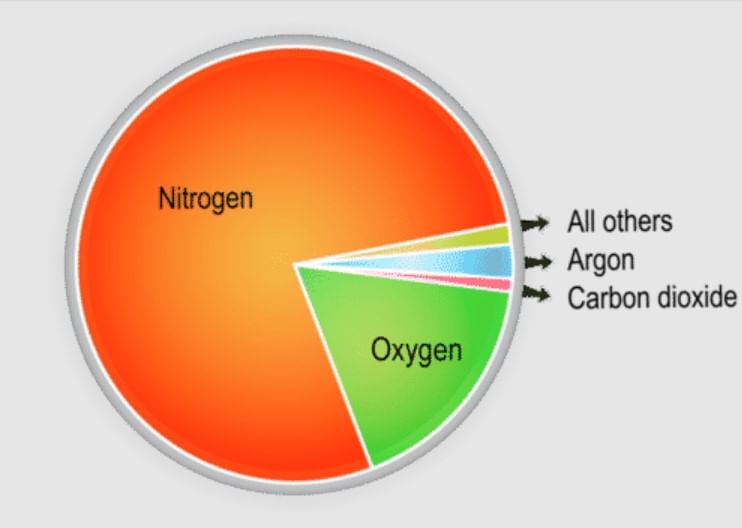
- Nitrogen accounts for 78% of total atmospheric volume. It is a relatively inert gas and is an essential constituent of all organic compounds. The primary function of nitrogen is to control combustion by diluting oxygen. It also indirectly helps in the oxidation of different kinds.
- Carbon Dioxide constitutes only about 0.038% of the dry air and is a product of combustion. Through photosynthesis, green plants absorb carbon dioxide from the atmosphere and use it to manufacture food and keep other biophysical processes going.
- Being an efficient absorber of heat, carbon dioxide is considered to be of great climatic significance. Carbon dioxide is considered to be a significant factor in the heat energy budget.
- With the increased burning of fossil fuels – oil, coal, and natural gas – the atmosphere's carbon dioxide percentage has been rising at an alarming rate.
- More carbon dioxide in the atmosphere means more heat absorption. This could significantly raise the temperature at lower levels of the atmosphere, thus inducing drastic climatic changes.
- The third important gas is Argon which constitutes only about 0.93%.
- Ozone (03) is another important gas in the atmosphere, a type of oxygen molecule consisting of three, instead of two, atoms. It forms less than 0.00006% by volume of the atmosphere and is unevenly distributed. It is between 20 km and 25 km altitude that the most significant concentrations of ozone are found. It is formed at higher altitudes and transported downwards.
- Ozone plays a crucial role in blocking the harmful ultraviolet radiation from the sun.
- Other gases found in almost negligible quantities in the atmosphere are neon, helium, hydrogen, xenon, krypton, methane, etc.
2. Water Vapour
- The vapor content in the atmosphere ranges from 0 to 4 % by volume.
- The atmospheric vapor is received through the evaporation of moisture and water from the water bodies (like seas and oceans, lakes, tanks and ponds, rivers, etc.), vegetation, and soil cover.
- Vapor depends on temperature, and therefore it decreases from the equator poleward in response to decreasing temperature towards the poles.
- The vapor content in the surface air in the moist tropical areas, at 50-degree and 70-degree latitudes, is 2.6%, 0.9%, and 0.2% (by volume) respectively.
- The content of vapour decreases upward.
- More than 90% of the total atmospheric vapour is found up to the height of 5 km.
- The moisture content in the atmosphere creates several forms of condensation and precipitation, e.g. clouds, fogs, dew, rainfall, frost, hailstorm, ice, snowfall, etc.
- Vapour is almost transparent for incoming shortwave solar radiation so that the electromagnetic radiation waves reach the earth’s surface without many obstacles. Still, the vapour is less transparent for outgoing shortwave terrestrial radiation. Therefore it helps in heating the earth’s surface and lower portion of the atmosphere because it absorbs terrestrial radiation.
3. Particulate Matter
- The Solid Particles present in the atmosphere consist of sand particles (from weathered rocks and also derived from volcanic ash), pollen grains, small organisms, soot, ocean salts; the upper layers of the atmosphere may even have fragments of meteors which got burnt up in the atmosphere.
- These particulates help in the absorbing, reflecting, and scattering of solar radiation, which adds the varied charming colour of red and orange at sunrise and sunset.
- The sky appears blue due to the selective scattering of solar radiation by dust particles.
- Salt particles become hygroscopic nuclei and thus help shape water drops, clouds and various forms of condensation and precipitation.
Question for Earth's Atmosphere: Composition & StructureTry yourself:Which gas accounts for the highest proportion in the atmosphere
View Solution
Structure of the Atmosphere
The atmosphere can be divided into different layers according to composition, density, pressure, and temperature variations.
Based on Composition
According to its composition, broadly it is divided into two layers-
- Homosphere
- Heterosphere
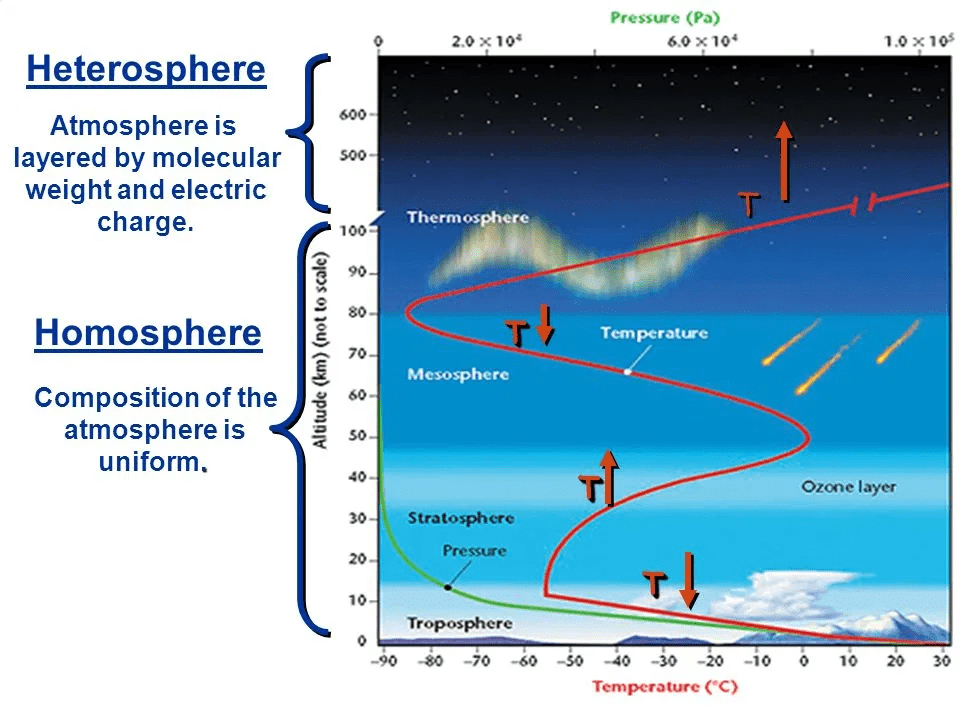
1. Homosphere
- In the Homosphere, there are three regions: The Troposphere, the Stratosphere, and the Mesosphere.
- Although air composition is the same throughout these three regions, the concentration of air decreases significantly with increasing altitude.
- Troposphere: The Troposphere is the earth’s weather layer. It contains nearly all weather conditions. As you go up in altitude, the temperature goes down. It is the bottom-most layer of the
- Stratosphere: The Stratosphere is the middle region of the Homosphere.
- Mesosphere: The Mesosphere is the top layer of the Homosphere.
- It extends from the earth’s surface up to an altitude of 80 km. Even though the atmosphere rapidly decreases in density with increasing altitude, the gases' composition remains uniform in the homosphere.
- The homosphere's exceptions are the concentration of Ozone (O3) in the stratosphere from almost 19-50 km and variation of water vapour and dust particles in the lower atmosphere. This uniform composition was attained approximately 600 million years ago.
2. Heterosphere
- In the Heterosphere, there are two regions: The Thermosphere and the Exosphere. These two regions are considered outer space. The gases in this layer are not evenly mixed. The Ionosphere overlaps the Mesosphere and the Thermosphere.
- Thermosphere: The thermosphere is the bottom region of the Heterosphere.
- Exosphere: The exosphere is the top region of the Heterosphere.
- The gases in this layer are not evenly mixed. It begins over 80km and extends up to 10,000 km.
- However, for all scientific purposes, the atmosphere's upper limit is taken as 480km as the earth’s gravitational pull becomes negligible after it.
- The atmosphere above it is called the exosphere, and it contains individual atoms of light gases like hydrogen, helium, etc.
Based on Change in Temperature:
Based on the temperature change, the atmosphere is broadly divided into five layers:
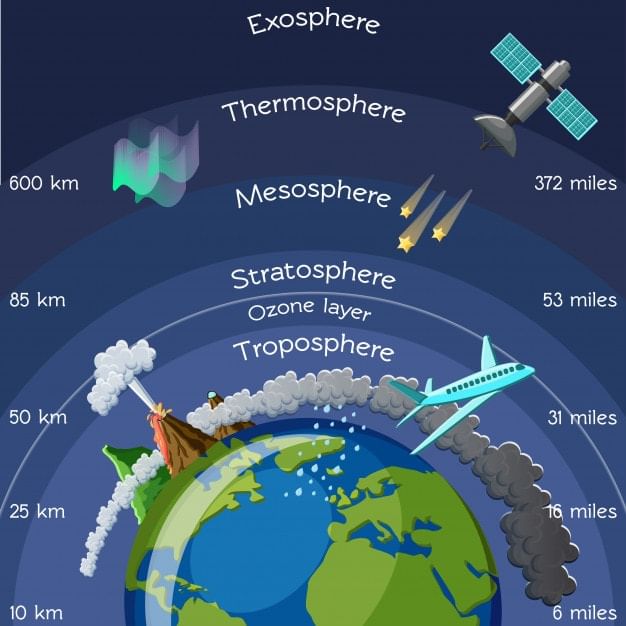
1. Troposphere
- It is the lowermost layer of the atmosphere. It extends up to 18 km at the equator, 13 km at mid-latitude, and about 8 km at poles.
- It contains approximately 90% of the total mass of the atmosphere.
- The entire weather phenomenon takes place in this layer. It contains all the water vapour, dust particles, clouds, etc.
- In the troposphere, the temperature decreases with an increase in height.
- The average rate of decrease of temperature with height is called a normal lapse rate, and it is equal to 6.4º C/km. The rate of decrease in temperature is not constant everywhere.
- The local rate of decrease is called the local lapse rate. The minimum temperature attained in this layer is -57 degrees C.
- Tropopause: It is the topmost layer of the troposphere. It acts as a boundary between the troposphere and stratosphere. Constant temperatures mark this layer.
2. Stratosphere
- It lies above the troposphere and extends uniformly across the globe up to 50 km.
- In this layer, the temperature increases with an increase in height. The temperature varies from -57 to 0 degrees C.
- The presence of the Ozonosphere characterizes this layer. Ozone is a highly reactive oxygen molecule made up of three atoms.
- Ozone absorbs the high-frequency ultraviolet radiation because of this absorption, the temperature of the layer increases.
- The energy absorbed is used in chemical reactions causing the formation of ozone gas.
- Ultraviolet rays are highly harmful to living organisms, including plants, animals as well as humans. Absorbing these radiations ozone layers make a protective layer around us.Question for Earth's Atmosphere: Composition & StructureTry yourself:The layer of the atmosphere which contains dust particles and water vapour is calledView Solution
3. Mesosphere
- The mesosphere extends from 50 – 80 km.
- The temperature decreases in this layer and reaches its minimum mark averaging -90o C although this temperature can vary.
- The homogenous layer extends up to the mesosphere. There is a layer of ions extending in the other layer at the upper boundary of the mesosphere. This layer of ions or charged particles helps reflect the radio waves and helps in telecommunication.
4. Thermosphere
- This is a region extending from 80 km to 480 km.
- It contains a functional ionosphere. The temperature rises very sharply in this layer as the gas molecules absorb the short wave radiations coming from the sun.
- The temperature can reach as high as 1200o C, but the thermosphere is not as ‘hot’ as we expect it to be despite such high temperatures.
- As the air density is so low in this layer, the energy is not easily transferred; hence the hotness is not felt.
5. Ionosphere
- This is the zone containing charged particles called ions. It lies from the upper mesosphere to the thermosphere.
- The charged particles are ionized by absorption of cosmic rays, gamma rays, X-rays, and shorter ultraviolet rays wavelengths.
- It is in this layer that incoming space vehicles and meteorites begin to heat due to friction.
- Above this layer, i.e. above 480 km, atomic oxygen is prevalent and beyond that first helium is more common, and then hydrogen atoms predominate.
- The ionosphere is a deep layer of electrically charged molecules and atoms (called ions) in the middle and upper mesosphere and the lower thermosphere, between about 60 and 400 kilometers (40 and 250 miles). The ionosphere is significant because it aids long-distance communication by reflecting radio waves to earth.
- It is also known for its Auroral displays, such as the “northern lights” that develop when charged atomic particles from the sun are trapped by earth's magnetic field near the poles. These particles “excite” the nitrogen molecules and oxygen atoms in the ionosphere, causing them to emit light, not unlike a neon light bulb.
The document Earth's Atmosphere: Composition & Structure | Geography for UPSC CSE is a part of the UPSC Course Geography for UPSC CSE.
All you need of UPSC at this link: UPSC
|
175 videos|619 docs|192 tests
|
FAQs on Earth's Atmosphere: Composition & Structure - Geography for UPSC CSE
| 1. What is the atmosphere? |  |
Ans. The atmosphere is a layer of gases surrounding a planet or other celestial body. It is held in place by the planet's gravity and plays a vital role in maintaining life on Earth.
| 2. Why is the atmosphere important? |  |
Ans. The atmosphere is important for several reasons. It protects the Earth from the harmful effects of the sun's ultraviolet radiation, regulates the planet's temperature, and provides the necessary gases for living organisms to survive. It also helps to distribute heat and moisture around the globe, influencing weather patterns.
| 3. What is the composition of the Earth's atmosphere? |  |
Ans. The Earth's atmosphere is primarily composed of nitrogen (about 78%) and oxygen (about 21%). Other gases present in smaller amounts include carbon dioxide, water vapor, and trace amounts of various gases such as argon, neon, helium, and methane.
| 4. How is the Earth's atmosphere structured? |  |
Ans. The Earth's atmosphere is divided into several layers. The lowest layer is the troposphere, where weather occurs and most of the Earth's air mass is concentrated. Above the troposphere is the stratosphere, which contains the ozone layer that absorbs harmful UV radiation. The mesosphere is above the stratosphere and is followed by the thermosphere, which contains the ionosphere. Finally, the exosphere is the outermost layer where the atmosphere gradually transitions into space.
| 5. What are the two ways to classify the Earth's atmosphere? |  |
Ans. The Earth's atmosphere can be classified based on its composition and based on changes in temperature. Based on composition, the atmosphere is divided into homosphere and heterosphere. The homosphere includes the troposphere, stratosphere, mesosphere, and most of the thermosphere, where the composition is relatively uniform. The heterosphere includes the upper part of the thermosphere and exosphere, where gases are stratified by molecular weight. Based on changes in temperature, the atmosphere is divided into different layers, each with its own temperature profile.
Related Searches





















