Electrochemical or Activity series | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Electrochemical |

|
| Characteristics of Electrochemical Series |

|
| Applications of Electrochemical Series |

|
| Solved Examples |

|
| Corrosion of Metals |

|
Electrochemical
- When the electrodes (metals and non-metals) in contact with their ions are arranged on the basis of the values of their standard reduction potentials or standard oxidation potentials, the resulting series is called the electrochemical or electromotive or activity series of the elements.
- By measuring the potentials of various electrodes versus standard hydrogen electrode (SHE), a series of standard electrode potentials has been established.
- By international convention, the standard potentials of electrodes are tabulated for reduction half reactions, indicating the tendencies of the electrodes to behave as cathodes towards SHE.
- Electrodes with positive E° values for reduction half reactions do in fact act as cathodes versus SHE, while those with negative E° values of reduction half reactions behave instead as anodes versus SHE. The electrochemical series is shown in the following table.
- A cell's standard state potential is the potential of the cell under standard state conditions, which is approximated with concentrations of 1 mole per liter (1 M) and pressures of 1 atmosphere at 25°C.
Standard Aqueous Electrode Potentials at 25°C 'The Electrochemical Series'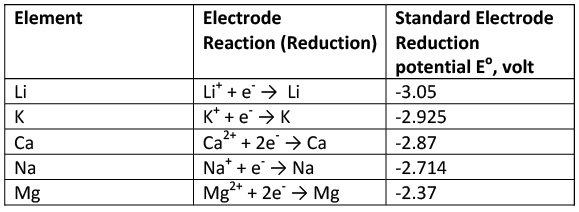

- The negative sign of standard reduction potential indicates that an electrode when joined with SHE acts as anode and oxidation occurs on this electrode.
- For example, standard reduction potential of zinc is -0.76 volt.
- When zinc electrode is joined with SHE, it acts as anode (-ve electrode) i.e., oxidation occurs on this electrode. Similarly, the +ve sign of standard reduction potential indicates that the electrode when joined with SHE acts as cathode and reduction occurs on this electrode.
Characteristics of Electrochemical Series
- The substances which are stronger reducing agents than hydrogen are placed above hydrogen in the series and have negative values of standard reduction potentials.
- All those substances which have positive values of reduction potentials and placed below hydrogen in the series are weaker reducing agents than hydrogen.
- The substances which are stronger oxidising agents than H+ion are placed below hydrogen in the series.
- The metals on the top (having high negative values of standard reduction potentials) have the tendency to lose electrons readily. These are active metals.
- The non-metals on the bottom (having high positive values of standard reduction potentials), have the tendency to accept electrons readily. These are active non-metals.
Applications of Electrochemical Series
1. Reactivity of Metals
The activity of the metal depends on its tendency to lose electron or electrons, i.e., tendency to form cation. This tendency depends on the magnitude of standard reduction potential. The metal which has high negative value (or smaller positive value) of standard reduction potential readily loses the electron or electrons and is converted into cation. Such a metal is said to be chemically active.
- The chemical reactivity of metals decreases from top to bottom in the series. The metal higher in the series is more active than the metal lower in the series. For example, Alkali metals and alkaline earth metals having high negative values of standard reduction potentials are chemically active. These react with cold water and evolve hydrogen. These readily dissolve in acids forming corresponding salts and combine with those substances which accept electrons.
- Metals like Fe, Pb, Sn, Ni, Co, etc., which lie a little down in the series do not react with cold water but react with steam to evolve hydrogen.
- Metals like Cu, Ag and Au which lie below hydrogen are less reactive and do not evolve hydrogen from water.
2. Electropositive Character of Metals
The electropositive character also depends on the tendency to lose electron or electrons. Like reactivity, the electropositive character of metals decreases from top to bottom in the electrochemical series. On the basis of standard reduction potential values, metals are divided into three groups:
- Strongly electropositive metals: Metals having standard reduction potential near about -2.0 volt or more negative like alkali metals, alkaline earth metals are strongly electropositive in nature.
- Moderately electropositive metals: Metals having values of reduction potentials between 0.0 and about -2.0 volt are moderately electropositive. Al, Zn, Fe, Ni, Co, etc., belong to this group
- Weakly electropositive metals: The metals which are below hydrogen and possess positive values of reduction potentials are weakly electropositive metals. Cu, Hg, Ag, etc., belong to this group.
3. To predict whether a given metal will displace another, from its salt solution
A metal higher in the series will displace the metal from its solution which is lower in the series, i.e., the metal having low standard reduction potential will displace the metal from its salt's solution which has higher value of standard reduction potential. A metal higher in the series has greater tendency to provide electrons to the cations of the metal to be precipitated.
Displacement of one nonmetal from its salt solution by another nonmetal: A nonmetal higher in the series (towards bottom side), i.e., having high value of reduction potential will displace another nonmetal with lower reduction potential i.e., occupying position above in the series. The nonmetal's which possess high positive reduction potentials have the tendency to accept electrons readily. These electrons are provided by the ions of the nonmetal having low value of reduction potential. Thus, Cl2 can displace bromine and iodine from bromides and iodides.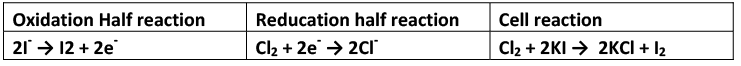
The activity or electronegative character or oxidising nature of the nonmetal increases as the value of reduction potential increases.
4. Displacement of hydrogen from dilute acids by metals
The metal which can provide electrons to H+ ions present in dilute acids for reduction, evolve hydrogen from dilute acids.
The metal having negative values of reduction potential possess the property of losing electron or electrons. Thus, the metals occupying top positions in the electrochemical series readily liberate hydrogen from dilute acids and on descending in the series tendency to liberate hydrogen gas from dilute acids decreases.
The metals which are below hydrogen in electrochemical series like Cu, Hg, Au, Pt, etc., do not evolve hydrogen from dilute acids.
- Displacement of hydrogen from water: Iron and the metals above iron are capable of liberating hydrogen from water. The tendency decreases from top to bottom in electrochemical series. Alkali and alkaline earth metals liberate hydrogen from cold water but Mg, Zn and Fe liberate hydrogen from hot water or steam.
5. Reducing Power of Metals
Reducing nature depends on the tendency of losing electron or electrons. More the negative reduction potential, more is the tendency to lose electron or electrons. Thus, reducing nature decreases from top to bottom in the electrochemical series. The power of the reducing agent increases as the standard reduction potential becomes more and more negative.
Sodium is a stronger reducing agent than zinc and zinc is a stronger reducing agent than iron. Alkali and alkaline earth metals are strong reducing agents
- Oxidising Nature of Nonmetals: Oxidising nature depends on the tendency to accept electron or electrons. More the value of reduction potential, higher is the tendency to accept electron or electrons. Thus, oxidising nature increases from top to bottom in the electrochemical series. The strength of an oxidising agent increases as the value of reduction potential becomes more and more positive.
F2 (Fluorine) is a stronger oxidant than Cl2, Br2 and I2.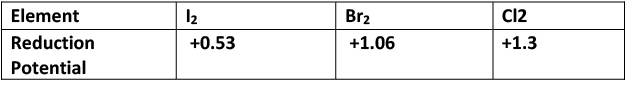
Products of Electrolysis
In case two or more types of positive and negative ions are present in solution, during electrolysis certain ions are discharged or liberated at the electrodes in preference to others. In general, in such competition the ion which is stronger oxidising agent (high value of standard reduction potential) is discharged first at the cathode.
The increasing order of deposition of few cations is: K+, Ca2+, Na+, Mg2+, Al3+, Zn2+, Fe2+, H+, Cu2+, Ag+, Au3+
Increasing Order of Deposition
Similarly, the anion which is stronger reducing agent (low value of standard reduction potential) is liberated first at the anode.
The increasing order of discharge of few anions is:
SO42-, NO3-, OH-, Cl-, Br-, I-
Thus, when an aqueous solution of NaCl containing Na+, Cl-, H+ and OH" ions is electrolysed, H+ ions are discharged at cathode and Cl- ions at the anode, i.e., H2 is liberated at cathode and chlorine at anode.
When an aqueous solution of CuS04 containing Cu2+, H+ and OH- ions is electrolysed, Cu2+ ions are discharged at cathode and OH- ions at the anode.
Cu is deposited on cathode while O2 is liberated at anode.
7. Calculation of Standard emf (E0) of Electrochemical Cell
The standard emf of the cell is the sum of the standard reduction potential of the two half cell: reduction half cell and oxidation half cell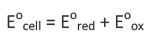
By convention, the standard oxidation potential is always expressed in terms of reduction potential.
Thus, standard oxidation potential (Eoox) = - standard reduction potential Eored Therefore, Eocell = ( standard reduction potential of reduction half cell) - ( standard reduction potential of oxidation half cell)
As oxidation takes place at anode and reduction takes place at the cathode. Hence, 
Example: For a reaction, 2Ag+ (aq) + Cd -> 2Ag + Cd+2(aq)
The standard reduction potential given are: Ag+/ Ag = 0.80 volt, Cd+2/ Cd = -0.40 volt
From the reaction, we can see that Cd losses electron and Ag+ gains. Hence, oxidation half cell or anode is Cd.
Using the formula,
8. Predicting the Feasibility of Redox Reaction
Any redox reaction would occur spontaneously if the free energy change (AG) is negative. The free energy is related to cell emf in the following manner:
AGo = nFEo
Where n is the number of electrons involved, F is the Faraday constant and Eo is the cell emf.
- AGo can be negative if Eo is positive.
- When Eo is positive, the cell reaction is spontaneous and serves as a source of electrical energy.
- If it comes out to be negative then the spontaneous reaction cannot take place.
- The resultant value of Eo for redox reaction is important in predicting the stability of a metal salt solution when stored in another metal container.
For example, let us find out whether we can store copper sulphate solution in a nickel vessel or not.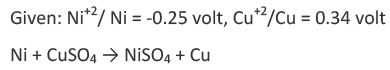
We want to see whether Ni metal will displace copper from copper sulphate solution to give NiSO4 by undergoing oxidation reaction.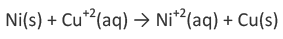
From the above reaction, it is clear oxidation terminal will be Ni electrode.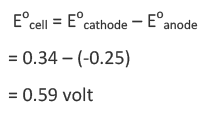
As the emf comes out to be positive, it implies copper sulphate reacts when placed in a nickel vessel and hence cannot be stored in it.
Solved Examples
Example 1: Predict whether the following reaction will occur spontaneously or not: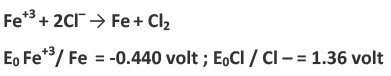
Ans:
Since chlorine has higher reduction potential than iron therefore at cathode reduction of chlorine occurs and oxidation of iron occurs at the anode.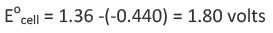
The positive value of E0Cell implies that reaction occurs spontaneously.
Example 2: The standard reduction potential at 250C for the following half-reaction are given below: 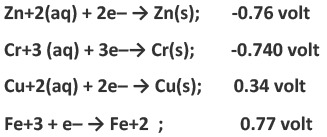
Which is the strongest reducing agent
Ans: Reducing agent is a chemical species that loses an electron to another chemical species in a redox chemical reaction. Since, reducing agent loses electron so it is oxidised. Out of the following given half-reaction, the reduction of Zn+2 has the lowest reduction potential(-0.762). We know that
Oxidation potential = -(reduction potential)
So in terms of standard oxidation potential Zinc will have the highest oxidation potential i.e, 0.762 volts. Therefore, zinc is the strongest reducing agent.
Example 3: The standard oxidation potential, E0 for the half-reactions are as follows,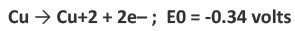
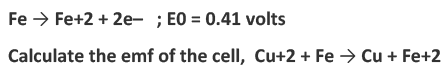
Ans: Eocell = (standard reduction potential of reduction half cell) - (standard reduction potential of oxidation half cell)
Eocell = -(standard oxidation potential of reduction half cell) - (-standard oxidation potential of oxidation half cell)
Eocell = -0.34-(-0.41)
Eocell = 0.07 volt.
Corrosion of Metals
Corrosion is defined as the deterioration of a substance because of its reaction with its environment. This is also defined as the process by which metals have the tendency to go back to their combined state, i.e., reverse of extraction of metals. In other words, Corrosion is the degradation of metals due to an electrochemical process. The formation of rust on iron, tarnish on silver, and the blue-green patina that develops on copper are all examples of corrosion.
The most familiar example of corrosion is the formation of rust on iron. Iron will rust when it is exposed to oxygen and water. The main steps in the rusting of iron appear to involve the following.
Once exposed to the atmosphere, iron rapidly oxidizes.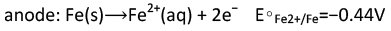
The electrons reduce oxygen in the air in acidic solutions.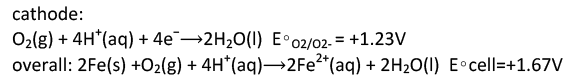
What we call rust is hydrated iron(IM) oxide, which forms when iron(II) ions react further with oxygen.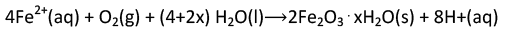
The number of water molecules is variable, so it is represented by x.
Several methods for protection of metals against corrosion have been developed. The most widely used are:
- Coating a metal surface with paint or enamel provides a barrier between the metal and the moisture in the environment.
- The process of coating a metal surface with another metal that is more likely to be oxidized is referred to as sacrificial coating. Ex: The corrosion-prone iron alloy steel is
- commonly coated with zinc, a more active metal, in a process known as galvanizing. Corrosion of the sacrificial zinc results in its oxidation; the iron is reduced, which renders it cathodic and inhibits its corrosion. Zinc is more easily oxidized than iron because zinc has a lower reduction potential. Even if the zinc coating is scratched, the zinc will still oxidize before the iron.
- Another important way to protect metal is to make it the cathode in a galvanic cell. This is cathodic protection and can be used for metals other than just iron. For example, the rusting of underground iron storage tanks and pipes can be prevented or greatly reduced by connecting them to a more active metal such as zinc or magnesium This is also used to protect the metal parts in water heaters. The more active metals (lower reduction potential) are called sacrificial anodes because they get used up as they corrode (oxidize) at the anode. The metal being protected serves as the cathode, and so does not oxidize (corrode). When the anodes are properly monitored and periodically replaced, the useful lifetime of the iron storage tank can be greatly extended.
FAQs on Electrochemical or Activity series - Chemistry Optional Notes for UPSC
| 1. What are the electrochemical characteristics of the electrochemical series? |  |
| 2. What are the applications of the electrochemical series? |  |
| 3. Can you provide some examples related to the electrochemical series? |  |
| 4. What is the significance of the electrochemical series in understanding corrosion of metals? |  |
| 5. How is the electrochemical series useful in battery design? |  |














