Electron Microscopic Appearance of a Skeletal Muscle | Medical Science Optional Notes for UPSC PDF Download
Overview
Muscle fibers are composed of myofibrils, which are further divided into individual filaments.
Myofilaments within the muscle contain multiple proteins, collectively forming the contractile machinery of the skeletal muscle.
The contractile mechanism in skeletal muscle is heavily reliant on proteins such as myosin-II, actin, tropomyosin, and troponin.
Troponin is comprised of three subunits: troponin I, troponin T, and troponin C.
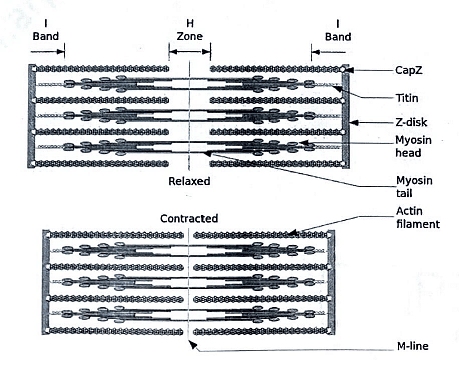
Each myofibril consists of interdigitating thick and thin filaments arranged longitudinally within sarcomeres.
Sarcomeres, the repeating units within myofibrils, contribute to the distinctive banding pattern observed in striated muscle.
A sarcomere extends from the Z line to another Z line, defining the structural and functional unit of muscle contraction.

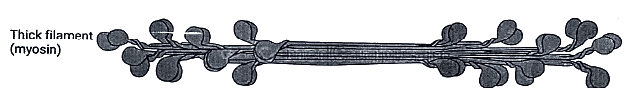
Thick Filaments
Found in the A band at the center of the sarcomere and are composed of myosin.
Myosin consists of six polypeptide chains, comprising one pair of heavy chains and two pairs of light chains.
Each myosin molecule possesses two "heads" attached to a single "tail."
The myosin heads play a crucial role in the process of muscle contraction, as they bind ATP and actin, participating in the formation of cross-bridges.
 Thick Filament
Thick Filament
Thin Filaments
Anchored at the Z lines and located in the I bands of the sarcomere.
Interdigitate with the thick filaments within a portion of the A band.
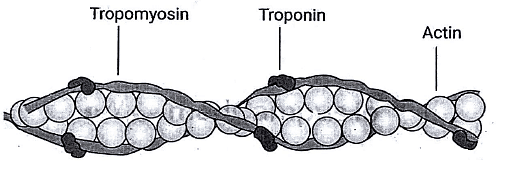
Composed of actin, tropomyosin, and troponin.
Troponin acts as the regulatory protein, allowing cross-bridge formation when it binds to Ca2+.
Troponin is a complex of three globular proteins:
- Troponin T ("T" for tropomyosin): Attaches the troponin complex to tropomyosin.
- Troponin I ("I" for inhibition): Inhibits the interaction of actin and myosin.
- Troponin C ("C" for Ca2+): The Ca2+-binding protein that, when bound to Ca2+, facilitates the interaction of actin and myosin.
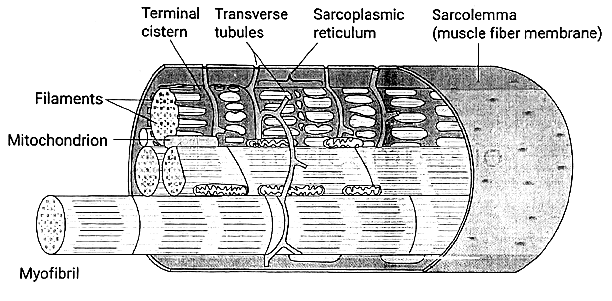
T Tubules
Form an extensive tubular network open to the extracellular space, facilitating the transmission of depolarization from the sarcolemmal membrane to the cell interior.
Positioned at the junctions of A bands and I bands.
Contain a voltage-sensitive protein known as the dihydropyridine receptor (DHPR).
Depolarization induces a conformational change in the dihydropyridine receptor.
Sarcoplasmic Reticulum
Internal tubular structure crucial for Ca2+ storage and release in excitation-contraction coupling.
Features terminal cisternae in intimate contact with T tubules, forming a triad arrangement.
Membrane includes the Ca2+-ATPase (Ca2+ pump) responsible for transporting Ca2+ from the intracellular fluid into the SR interior, maintaining low intracellular [Ca2+].
Contains a Ca2+ release channel called the ryanodine receptor.

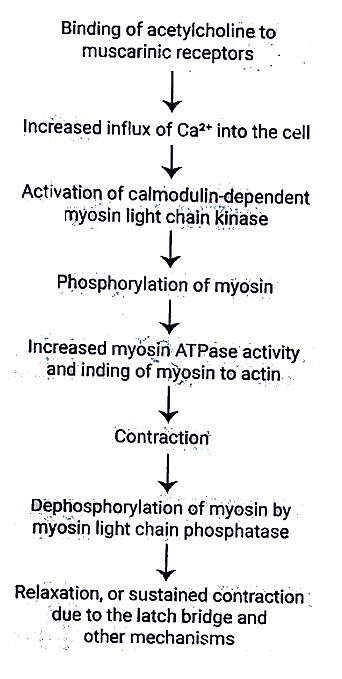
Latch Phenomenon in Smooth Muscle
Dephosphorylation of myosin light chain kinase: Does not always result in smooth muscle relaxation.
Latch Bridge Mechanism: Myosin cross-bridges stay attached to actin even after the cytoplasmic Ca2+ concentration decreases.
Outcome: Sustained contraction with minimal energy expenditure, particularly crucial in vascular smooth muscle.
Relaxation: Presumably happens when the Ca2+-calmodulin complex eventually dissociates.

There is no troponin; instead, Ca2+ regulates myosin on the thick filaments.
Nerve muscle physiology-Repeats
Muscle physiology
Q1: Differentiate between isometric and isotonic contraction in skeletal muscle. Give one example of each type. (2015)
Q2: What are modes of contraction in a skeletal muscle? Give suitable examples. Explain length-tension relationship in skeletal muscle. (2018)
Q3: Name the contractile proteins in skeletal muscle. What is the electron microscopic appearance of muscle? Write sequence of events in muscular contraction. (2011)
Q4: Compare with the help of a diagram the length-tension relationship for skeletal, cardiac and smooth muscles. What is the latch phenomenon in smooth muscle (2001)?
|
7 videos|219 docs
|
FAQs on Electron Microscopic Appearance of a Skeletal Muscle - Medical Science Optional Notes for UPSC
| 1. What is the role of the sarcoplasmic reticulum in muscle contraction? |  |
| 2. What is the latch phenomenon in smooth muscle? |  |
| 3. How does the electron microscopic appearance of skeletal muscle differ from smooth muscle? |  |
| 4. How does the sarcoplasmic reticulum regulate muscle contraction? |  |
| 5. What are the implications of the latch phenomenon in smooth muscle? |  |