F block: Oxidation States and their Stability | Chemistry Optional Notes for UPSC PDF Download
Oxidation States and their Stability
- The oxidation states of Ln are based on their electronic configuration.
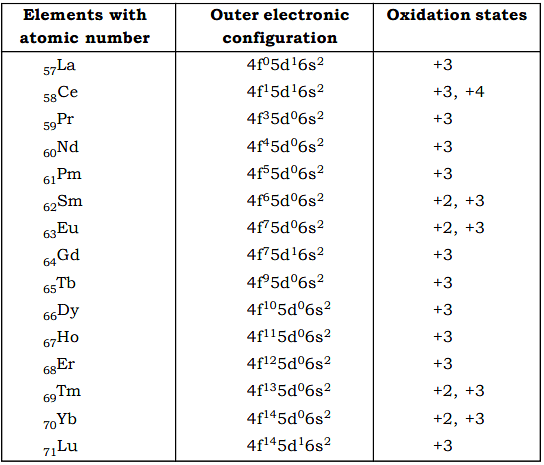
- The principal oxidation state of all the Ln elements is +3. Cesium shows +4 oxidation state, which is stable in solutions as well. +2 oxidation state is stable in Eu and Yb due to 4f7 (half filled) and 4f14 (filled) electronic configuration. Sm and Tm also show +2 oxidation state in some cases.
- The common +3 oxidation state for all Ln appears to be a consequence of greater stabilization of 4f orbitals in comparison to 5d or 6s with increasing ionic charge. The order of penetration of orbitals into the inner electron core decreases as 4f > 5d > 6s. As successive ionization increases the net charge on the Lanthanide cation, the 4f electrons are affected most, i.e, their energy is lowered to the greatest extent. In Ln3+ ion, the 4f electrons are thus stabilised to a greater extent than the 5d or 6s electrons, so these latter electrons usually ionise in most cases.
- The formation and stabilization of any ion in a particular oxidation state may be depicted in a relevant Born–Haber cycle of several enthalpy terms like sublimation, ionization, hydration of the ion etc. The oxidation states of Ln are thus collective effects of several factors.
- The oxidation states of actinides are also based basically on its electronic configuration.

Unlike the lanthanides, actinides offer a number of oxidation states, at least two oxidation states being found for most actinides.
This may be due to many reasons as given below:
- This is due to the proximity in successive ionization energies, but for higher oxidation states where covalent bonding is most likely, other factors needs consideration.
- The 5f orbitals have a longer spatial extension than the 4f orbitals and can participate better in covalent bonding.
- The energy differences between 5f, 6d and 7s orbitals may often be overcome by chemical binding energies, justfying their involvement in higher oxidation states.
There are several features of oxidation states in actinides:
- For elements up to U, the stable oxidation state involve all the valence electrons.
- Np also shows oxidation state of +7 involving all the valence electrons, but its stable state is +5.
- Pu and Am also shows oxidation of +7 and +6, but the stable oxidation state of Pu is +4 and that Am is +3.
- Rest of the actinides have their stable oxidation state as +3.
- The importance of half filled 5f orbital in Am, Cm and Bk is there as in the case of lanthanides.
The overall pattern resemble ‘d’ block elements where the valence electrons are fully utilized in the most stable oxidation states until middle of the series, after which it becomes stabilised in nature. Thus +3 oxidation state becomes stabilised only in the later actinides.
FAQs on F block: Oxidation States and their Stability - Chemistry Optional Notes for UPSC
| 1. What are oxidation states and why are they important in the f block of elements? |  |
| 2. How do oxidation states of f block elements affect their stability? |  |
| 3. Can f block elements exhibit multiple oxidation states? |  |
| 4. How does the stability of oxidation states in f block elements affect their reactivity? |  |
| 5. How can the knowledge of oxidation states in f block elements be applied in real-life scenarios? |  |





















