|
What is the principle behind Lassaigne's test for detecting nitrogen in organic compounds? |
Card: 1 / 30 |
|
Lassaigne's test involves fusing the organic compound with sodium metal, then testing the sodium extract with Nessler's reagent. The formation of a brown precipitate indicates the presence of ammonium ions (NH₄⁺), confirming nitrogen. |
Card: 2 / 30 |
|
Fill in the blanks: Sulphur is detected in organic compounds by the reaction of the sodium extract with ___, resulting in a ___ precipitate. |
Card: 3 / 30 |
|
True or False: The presence of halogens in organic compounds can be confirmed by the formation of a white precipitate with silver nitrate. |
Card: 5 / 30 |
|
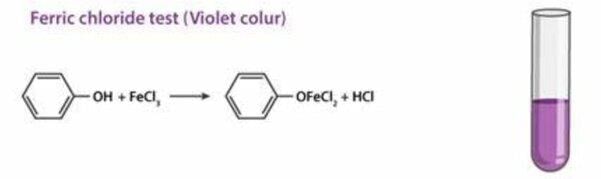
What color indicates the presence of phenolic compounds when tested with ferric chloride? |
Card: 7 / 30 |
|
Riddle: I am a test that turns a solution blue for amino acids, but when I react with acetic acid, I turn red. What am I? |
Card: 9 / 30 |
|
The carboxyl group is detected by adding sodium bicarbonate, which releases carbon dioxide gas, indicated by effervescence, confirming the presence of a carboxyl group. |
Card: 12 / 30 |
|
Mohr's salt is prepared by reacting ferrous sulfate (FeSO₄) with ammonium sulfate ((NH₄)₂SO₄) in the presence of excess dilute sulfuric acid, forming (NH₄)₂Fe(SO₄)₂ and water. |
Card: 14 / 30 |
|
Fill in the blank: The preparation of acetanilide involves the reaction of aniline with ___ to form C₆H₅NHCOCH₃. |
Card: 15 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
What occurs in the titration of oxalic acid with potassium permanganate in acidic medium? |
Card: 17 / 30 |
|
The reaction produces a color change, resulting in the formation of carbon dioxide and manganese ions, indicating the endpoint of the titration. |
Card: 18 / 30 |
|
True or False: A yellow precipitate in qualitative salt analysis indicates the presence of lead ions (Pb²⁺). |
Card: 19 / 30 |
|
Riddle: I am a black precipitate formed when sulfide ions react with lead acetate. What am I? |
Card: 23 / 30 |
|
Fill in the blanks: The enthalpy change during the dissolution of CuSO₄ in water is measured as the ___ of solution. |
Card: 25 / 30 |
|
Explain how a strong acid-strong base titration reaches its endpoint using a pH indicator. |
Card: 27 / 30 |
|
The endpoint is reached when the pH indicator changes color, indicating that the acid has completely reacted with the base to form water and a salt. |
Card: 28 / 30 |
|
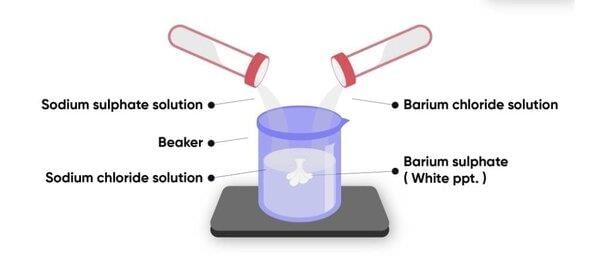
What is the reaction that confirms the presence of sulfate ions (SO₄²⁻) during qualitative analysis? |
Card: 29 / 30 |
|
The reaction with barium chloride (BaCl₂) forms a white precipitate of barium sulfate (BaSO₄), confirming the presence of sulfate ions. |
Card: 30 / 30 |