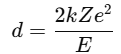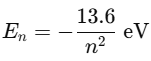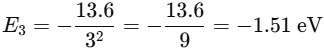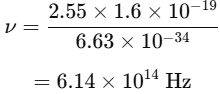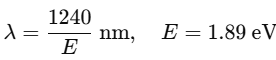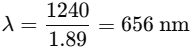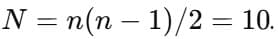|
The atom ionizes—the electron completely escapes, and the atom is no longer neutral! |
Card: 4 / 22 |
|
Energy levels are quantized, meaning electrons can only exist in fixed orbits where their wave fits perfectly. |
Card: 6 / 22 |
|
The Balmer series appears in the visible spectrum. What’s the color of the longest wavelength transition? |
Card: 7 / 22 |
|
An alpha particle (charge = +2e) moves toward a gold nucleus (Z = 79) with 7.7 MeV energy. |
Card: 9 / 22 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
An electron in hydrogen jumps from n = 4 to n = 2. The energy released is 2.55 eV. Find the emitted photon's frequency using: |
Card: 13 / 22 |
|
Find the wavelength of the emitted photon for the n = 3 to n = 2 transition. Use: |
Card: 15 / 22 |
|
Number of possible spectral lines when an electron drops from n=5n=5n=5 to ground state (all paths allowed): A) 4 |
Card: 19 / 22 |