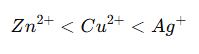|
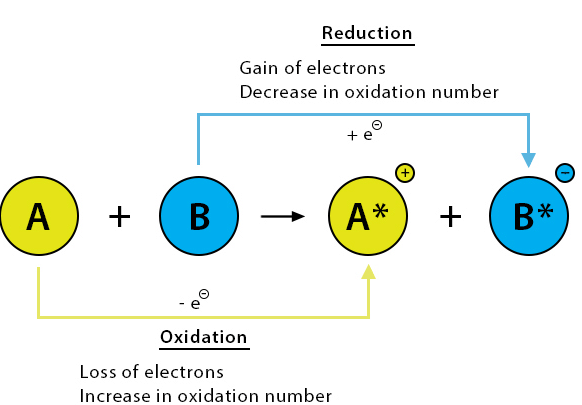
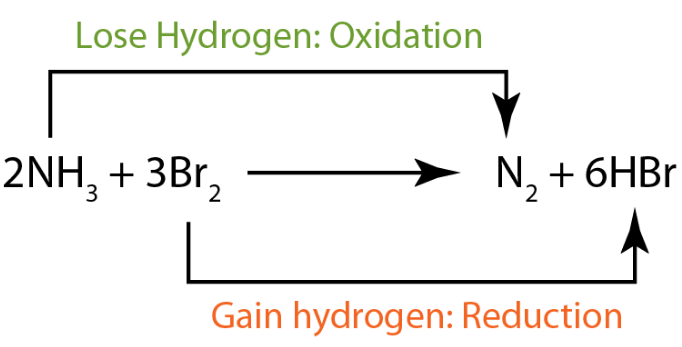
Redox reactions are chemical reactions in which oxidation and reduction occur simultaneously. |
Card: 2 / 44 |
|
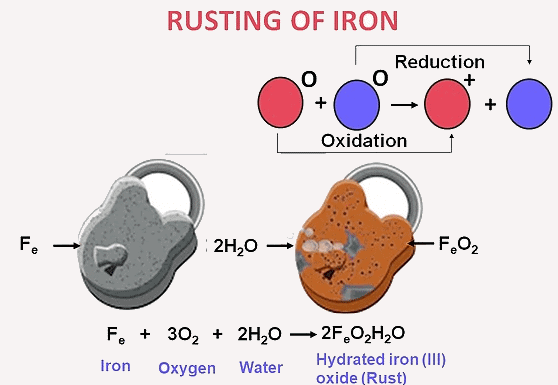
1) Rusting of iron: 4Fe + 3O2 + 6H2O → 4Fe(OH)3 2) Combustion of fuels: CH4 + 2O2 → CO2 + 2H2O
|
Card: 4 / 44 |
|
A substance that gains electrons and gets reduced. Example: Oxygen, chlorine, nitric acid. |
Card: 18 / 44 |
|
A substance that loses electrons and gets oxidized. Example: Hydrogen, carbon, sodium. |
Card: 20 / 44 |
|
The oxidation number is the charge an atom would have if all bonds were purely ionic. |
Card: 22 / 44 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Card: 24 / 44 |
|
Oxygen = -2, so: 2x + 3(-2) = 0 2x - 6 = 0 x = +3 Thus, Fe has an oxidation number of +3. |
Card: 26 / 44 |
|
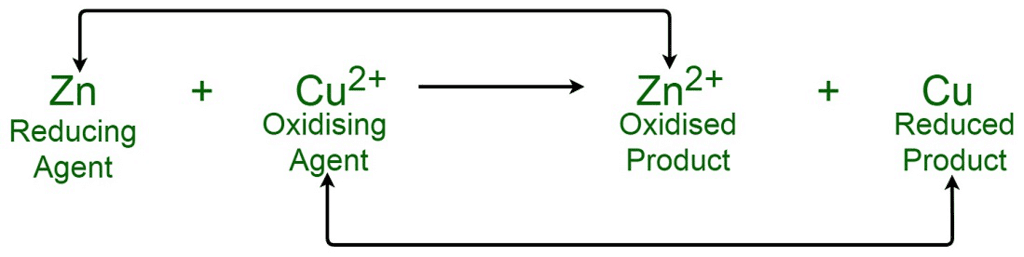
Combination reactions – Two substances combine to form one. C + O2 → CO2 Decomposition reactions – A single substance breaks into two or more. 2H2O2 → 2H2O + O2 Displacement reactions – One element replaces another in a compound. Zn + CuSO4 → ZnSO4 + Cu Disproportionation reactions – One element is oxidized and reduced in the same reaction. 2H2O2 → 2H2O + O2 |
Card: 28 / 44 |
|
Oxidation Number Method – Change in oxidation numbers is balanced. Half-Reaction Method – Oxidation and reduction half-reactions are balanced separately. |
Card: 30 / 44 |
|
Balance the reaction using the oxidation number method: |
Card: 31 / 44 |
|
Identify oxidation states: Cr₂O₇²⁻ (Cr = +6) → Cr³⁺ (Cr = +3) Fe²⁺ → Fe³⁺ Oxidation number changes: Cr⁶⁺ decreases by 3 per atom (total 6 for 2 Cr). Fe²⁺ increases by 1 per atom. Balance charge using electrons and H⁺ ions: Cr₂O₇²⁻ + 6Fe²⁺ + 14H⁺ → 2Cr³⁺ + 6Fe³⁺ + 7H₂O |
Card: 32 / 44 |
|
Respiration: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy Corrosion of metals: Fe + O₂ + H₂O → Fe₂O₃ ⋅ xH₂O Photosynthesis: 6CO₂ + 6H₂O + sunlight → C₆H₁₂O₆ + 6O₂ |
Card: 34 / 44 |
|
A redox titration determines the amount of an oxidizing or reducing agent in a solution using a redox indicator. |
Card: 36 / 44 |
|
It is the voltage associated with a reduction reaction at standard conditions (1M, 1 atm, 25°C). |
Card: 40 / 44 |
|
Arrange the following in order of increasing oxidizing power: Zn²⁺, Cu²⁺, Ag⁺. |
Card: 43 / 44 |
|
Oxidizing power depends on the standard reduction potential (E°)—a higher E° means a stronger oxidizing agent.
Since oxidizing power increases with E°, the order is: |
Card: 44 / 44 |