Group-13 Elements: Boron Family | Chemistry Class 12 - NEET PDF Download
What are Group 13 Elements?
The group 13 elements are the first group in the p-block of the periodic table.
All the elements of group 13 are also called the boron family. The periodic table is segregated into s, p, d and f-blocks. This segregation is done based on the valence electron, if the valence electron falls on the p subshell, it comes in p-block and so on.
 Group 13 Elements
Group 13 Elements
The members of Group 13 elements are:
- Boron
- Aluminium
- Gallium
- Indium
- Thallium
The general electronic configuration for the group 13 elements is ns2np1. Al is the most abundant metal and third most abundant element in the earth’s crust.
Physical Properties & Trends of Group 13 Elements
1. Electronic Configuration
- Their valence shell electronic configuration of this group is ns2np1. Here is the detailed electronic configuration of all the elements.
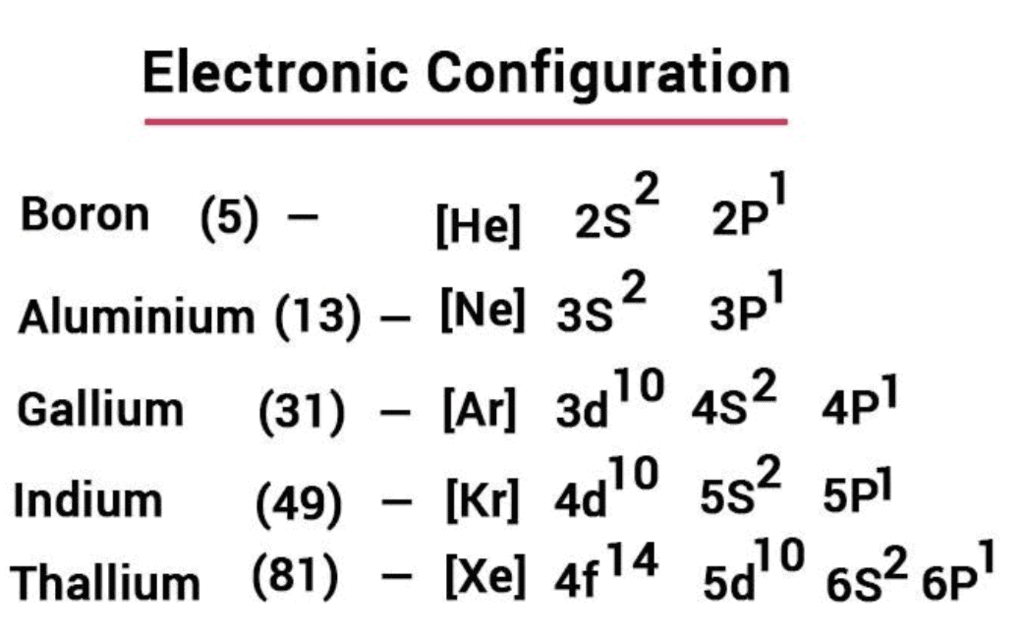
2. Atomic Radii and Ionic Radii
- Group 13 elements have a smaller size than those of alkaline earth metals due to greater effective nuclear charge, Zeff’

- Atomic radii increase on going down the group with an anomaly at gallium (Ga). Atomic radius increases down the group (Tl has the largest atomic radius.) An unexpected decrease in the atomic size of Ga is due to the presence of electrons in d-orbitals which do not screen the attraction of the nucleus effectively. The ionic radii regularly increase from B3+ to TI3+.
3. Density
It increases regularly on moving down the group from B to Tl.
4. Melting and Boiling Points
- The Melting point and boiling point of group 13 elements are much higher than those of group 2 elements.
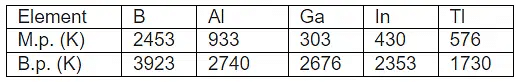
- The melting point decreases from B to Ga and then increases, due to structural changes in the elements. Boron has a very high melting point because of its three-dimensional structure in which B atoms are held together by strong covalent bonds.
- The low melting point of Ga is due to the fact that it consists of Ga2 molecules, and Ga remains liquid upto 2276 K. Hence, it is used in a high-temperature thermometer.Question for Group-13 Elements: Boron FamilyTry yourself:Which of the following element has the highest melting point?View Solution
5. Ionisation Enthalpy (IE)
- The initial ionization enthalpy values of Group 13 elements are comparatively lower than those of the corresponding alkaline earth metals. This can be attributed to the ease of electron removal, especially considering the [ns2 np1 configuration].
- The trend of decreasing ionization energy persists as one moves down the group. This is primarily due to the electrons being farther from the core, making them easier to remove. However, it's worth noting that thallium (Tl) does not conform to this trend.
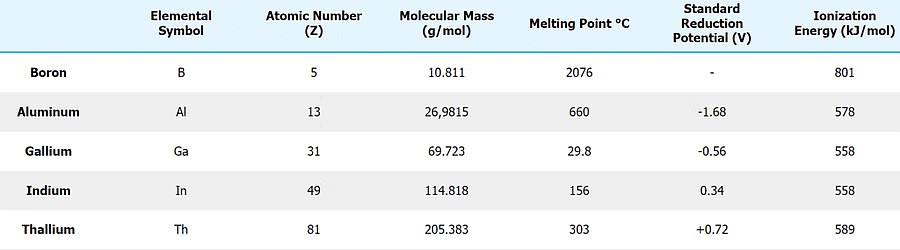 Trends & Properties of Group 13 elements
Trends & Properties of Group 13 elements
- Upon descending the group, the ionization enthalpy decreases from boron (B) to aluminum (Al). Nevertheless, gallium (Ga), the subsequent element, exhibits a slightly higher ionization enthalpy than Al.
- This anomaly can be attributed to the inefficient shielding provided by intervening d-electrons. The trend then resumes with a decrease in ionization enthalpy for indium (In) before concluding with an increase in the last element, thallium (Tl).
6. Oxidation States
- B and Al show an oxidation state of +3 only while Ga, In and Tl exhibit oxidation states of both +1 and +3.
- As we move down in group 13, due to the inert pair effect the tendency to exhibit +3 oxidation state decreases and the tendency to attain +1 oxidation state increases. Stability of +1 oxidation state follows the order Ga < In < Tl.
- Inert pair effect is the reluctance of the s-electrons of the valence shell to take part in bonding. It occurs due to poor shielding of the ns2 – electrons by the intervening d and f – electrons. It increases down the group and thus, the lower elements of the group exhibit lower oxidation states.
7. Electropositive (Metallic) Character
- These elements are less electropositive than the alkaline earth metals due to their smaller size and higher ionisation enthalpies.
- On moving down the group, the electropositive character first increases from B to Al and then decreases from Ga to Tl, due to the presence of d and f-orbitals which causes poor shielding.
8. Reducing Character
- It decreases down the group from Al to Tl because of the increase in electrode potential value for M3+ / M.
- Therefore, it follows the order: AI > Ga > In > Tl
9. Complex Formation
Due to their smaller size and greater charge, these elements have a greater tendency to form complexes than the s-block elements.
10. Nature of Compounds
The tendency of the formation of ionic compounds increases from B to Tl. Boron forms only covalent compounds whereas Al can form both covalent as well as ionic compounds. Gallium forms mainly ionic compounds, although anhydrous GaCI3 is covalent.
Chemical Properties of Group 13 Elements
Group 13 elements, also known as the boron group, exhibit a variety of chemical properties that are influenced by their electronic configurations and position on the periodic table. Here are some notable chemical properties of Group 13 elements:
1. Action of Air
- Crystalline boron is unreactive whereas amorphous boron is reactive. It reacts with air at 700°C as follows-
4B + 3O2 → 2B2O3
2B + N2 → 2BN - Al is stable in air due to the formation of the protective oxide film.
4Al + 3O2 → 2Al2O3 - Thallium is more reactive than Ga and In due to the formation of unipositive ion, Tl+.
4Tl + O2 → 2Tl20
2. Reaction with Nitrogen
The chemical reaction of boron and Aluminium with nitrogen at a high temperature is as follows :
3. Action of Water
- Both B and Al do not react with water but amalgamated aluminium reacts with H2O evolving H2.
2Al(Hg) + 6H2O → 2AI(OH)3 + 3H2 + 2Hg - Ga and In do not react with pure cold or hot water but Tl forms an oxide layer on the surface.
4. Reaction with Alkalies
- Boron dissolves in alkalies and gives sodium borates.

- Aluminium also reacts with alkali and liberates hydrogen.
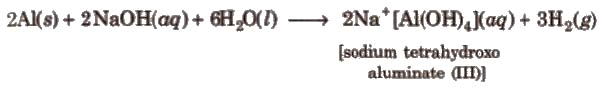
5. Reaction with Carbon
Aluminium carbide is ionic and forms methane with water.
6. Hydrides
- Elements of group 13 do not combine directly with H2 to form hydrides, therefore their hydrides have been prepared by indirect methods, e.g
 Boron forms a number of hydrides, they are known as boranes. Boranes catch fire in the presence of oxygen.B2H6 + 3O2 → B2O3 + 3H2O; & ΔcH° = – 1976 kJ mol-l
Boron forms a number of hydrides, they are known as boranes. Boranes catch fire in the presence of oxygen.B2H6 + 3O2 → B2O3 + 3H2O; & ΔcH° = – 1976 kJ mol-l- Boranes are hydrolysed by water.
B2H6 + 6H2O → 2H3BO3 + 6H2 - Boranes are stable but the stability of hydrides of Al, Ga, In, and Tl decreases on moving down the group because the strength of the M-H bond decreases.
Structure of diborane:
- BH3 does not exist as such, but exists as a dimer, i.e; B2H6(diborane].
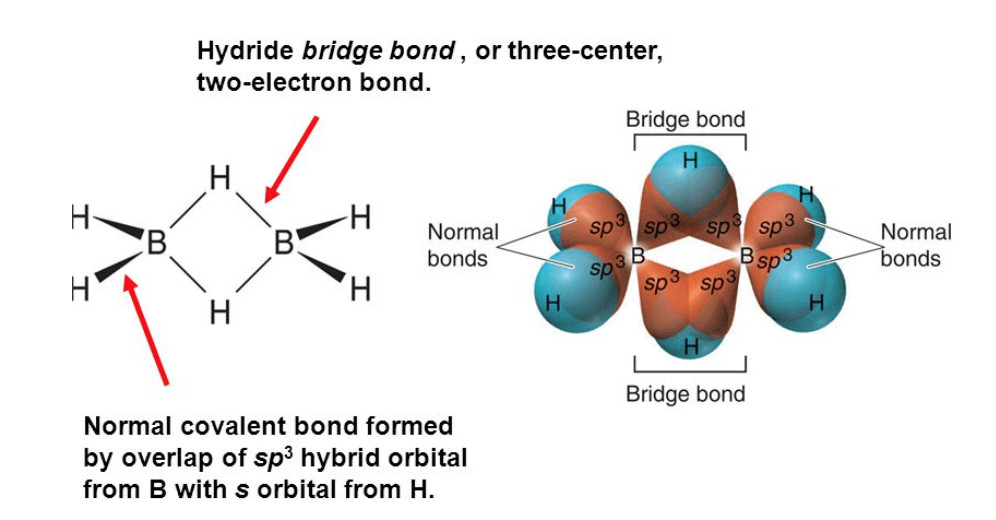
- In the above structure, B atoms are in sp3 – hybrid state. There are six B-H bonds out of which four B-H bonds are normal bonds present in the same plane while rest two B-H bonds behave as bridge bonds, ie; 3c – 2e (three centre-two electrons, also known as a banana bond) and present above and below the plane of the molecules which do not have a sufficient number of electrons to form covalent bonds.
- Aluminium (Al) forms a polymeric hydride of general formula (AlH3)x which decomposes into its elements on heating.
7. Oxides
- Except for Tl, all the elements of group 13 form oxides or general formula M2O3 on heating with oxygen.
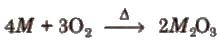
- Tl forms thallium (l) oxide. Tl2O is more stable than thallium (III) oxide TI2O3 due to the inert pair effect.
8. Nature of Oxides and Hydroxides
- B(OH)3 or H3BO3 is soluble in water, while other hydroxides are insoluble in water.
- On moving down the group, there is a change from acidic to amphoteric and then to the basic character of oxides and hydroxides or group 13 elements.
9. Halides
- All the elements of the boron family (except Tl) form trihalides of type MX3.
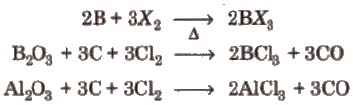
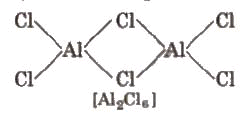
- All the boron trihalides [(BX3) and aluminium trihalides AlX3 (except AIF3 which is ionic) are covalent compounds. AlX3 exists as a dimer while BX3 is a monomer because the boron atom is too small to coordinate with four large halide ions.
- The energy released during the formation of the bridge structure is not sufficient for the cleavage of the typical pπ – pπ bond in BF3.
- BF3 is a colourless gas, BCl3 and BBr3 are colourless fuming liquids and BI3 is a white solid at room temperature.
- Trihalides of group 13 elements behave as Lewis acids because of their strong tendency to accept a pair of electrons. The relative strength of Lewis acids of boron trihalides is: BF3 < BCI3, < BBr3, < BI3.
This is due to pπ – pπ back bonding in BF3 which makes it less electron deficient. - The halides of group 13 elements behave as Lewis acids and the acidic character is BX3 > AIX3 > GaX3 > InX3 (where, X = Cl, Br or I)
TICI3 decomposes to TICl and Cl2 and hence acts as an oxidising agent.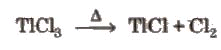
Anomalous Behaviour of Boron
Boron shows anomalous behaviour with the other members of the group, due to the following reasons:
- The smallest size in the group.
- High ionisation energy.
- Highest electronegativity in the group.
- Absence of vacant d-orbital.
Few Points of Difference
- It is non-metal while other members of the group are metallic.
- It shows allotropy while other members do not.
- It has the highest melting point and boiling point in group 13.
- It forms only covalent compounds while other members form both ionic and covalent compounds.
- The halides of boron exist as monomers while AlCl3 exists as a dimer.
- The oxides and hydroxides of boron are weakly acidic while those of aluminium are amphoteric and those of other elements are basic.
- It can be oxidised by concentrated HNO3 while aluminium becomes passive due to the formation of an oxide layer on the surface.
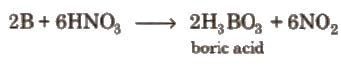
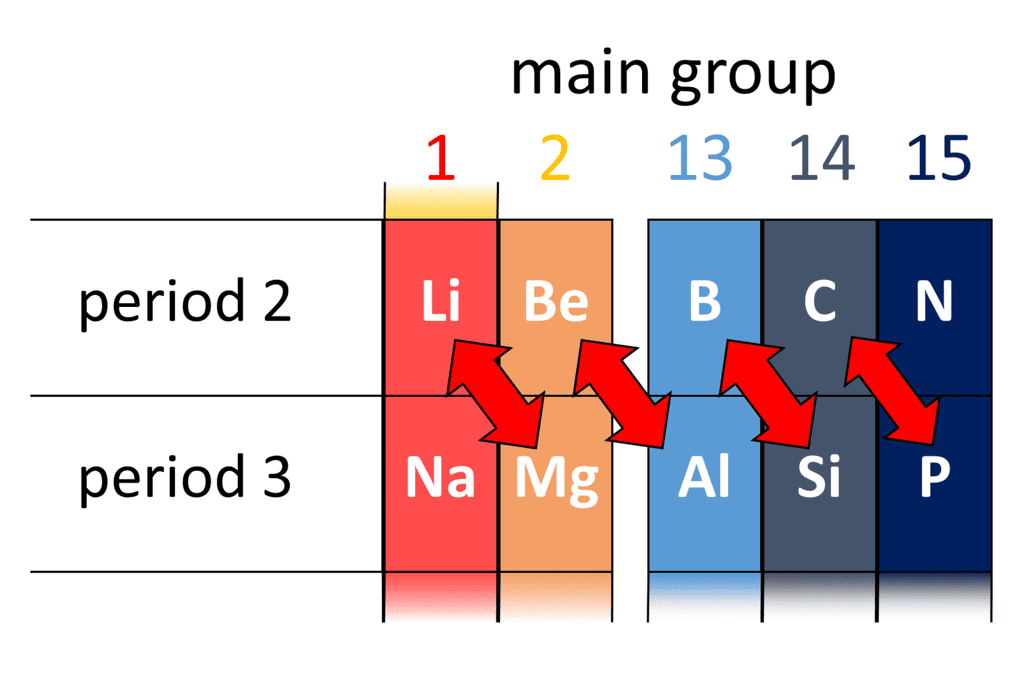
Diagonal Relationship between Boron and Silicon
Boron exhibit resemblance with its diagonal element silicon of group 14.
 Diagonal Relationship
Diagonal Relationship
- Both Band Si are non-metals.
- Both are semi-conductors.
- Both Band Si form covalent hydrides, i.e.. boranes and silanes respectively.
- Both form covalent, and volatile halides which fume in moist air due to the release of HCI gas.
BCI3 + 3H2O → H3 BO3 + 3HCl
SiCl4 + 4H2O → Si(OH)4 + 4HClQuestion for Group-13 Elements: Boron FamilyTry yourself:Which of the following property is not a similarity between Boron and silicon?View Solution
- Both form solid oxides which get dissolve in alkalies forming borates and silicates respectively.
- Both react with electropositive metals and give binary compounds, which yield a mixture of boranes and silanes on hydrolysis.Question for Group-13 Elements: Boron FamilyTry yourself: Boron shows a diagonal relationship with ____________View Solution
Metallurgy of Boron
Occurrence
It does not occur in a free state. Its important minerals are
- Borax (or Tineal), Na2B4O7 * 1OH2O
- Kernite, Na2B4O7 * 4H2O
- Orthoboric acid, H3BO3
Isolation
Elemental boron is obtained by the following methods:
- By reduction of boric oxide with highly electropositive metals like K, Mg, AI, Na etc, in the absence of air.
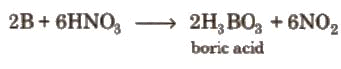
- By the reaction of boron halides with hydrogen.

Uses of Boron
- As a semi-conductor.
- Boron steel rods are used to control nuclear reactions.
5B10 + 0n1 → 5B11Question for Group-13 Elements: Boron FamilyTry yourself:Which of the following is not a use of Boron?View Solution
Compounds of Boron
1. Borax or Sodium Tetraborate Decahydrate[Na2B4O7.1OH2O]
Preparation
It occurs naturally as tineal in dried up lakes. It is obtained by boiling mineral colemanite with a solution of Na2CO3.
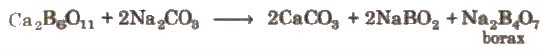 NaBO2 can be removed by passing CO2 through it.
NaBO2 can be removed by passing CO2 through it.4NaBO2 + CO2 → Na2CO3 + Na2B4O7
Properties
(i) Its aqueous solution is basic in nature.
Na2B4O7 + 7H2O → 2NaOH + 4H3BO3
(ii) On heating with ethyl alcohol and conc. H2SO4. It gives volatile vapours of triethyl borate which burn with a green flame.
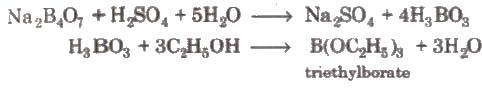
(iii) Action of heat:
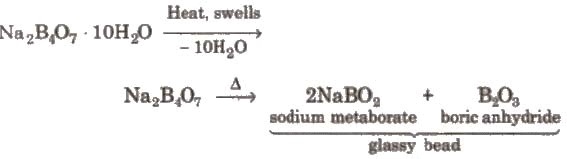 Borax bead is used for the detection of coloured basic radicals under the name borax bead test e.g.,
Borax bead is used for the detection of coloured basic radicals under the name borax bead test e.g., 2. Boric Acid or Orthoboric Acid [H3BO3 or B(OH)3]
2. Boric Acid or Orthoboric Acid [H3BO3 or B(OH)3]
Preparation
By treating borax with dil. HCl or dil. H2SO4.
Na2B4O7 + 2HCl + 5H2O → 2NaCI + 4H3BO3
Properties
(i) It is a weak monobasic acid (Lewis acid).
H3BO3 + 2H2O → [B(OH)4]– + H3O+
(ii) With C2H5OH and cone H2SO4, it gives triethyl borate.
It is used as an antiseptic and eye lotion under the name ‘boric lotion’, and as a food preservative.3. Borazine or Borazole, [B3N3H6]It is a colourless liquid having a six-membered ring of alternating B and N atoms. It is also called ‘inorganic benzene’. It is prepared by B2H6 as follows:
 The π electrons in borazine are only partially delocalised. It is more reactive than benzene.
The π electrons in borazine are only partially delocalised. It is more reactive than benzene.Compounds of Aluminium
1. Anhydrous Aluminium Chloride [AlCl3 or Al2Cl6]
Preparation
(i) It cannot be prepared by heating AICI3. 6H2O.
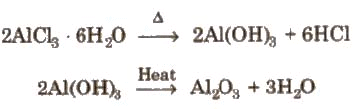 (ii) By passing dry chlorine or HCl gas over heated Al.
(ii) By passing dry chlorine or HCl gas over heated Al.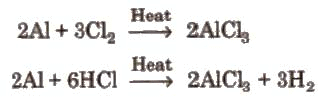 (iii) By heating a mixture of alumina and carbon in a current of dry chlorine.
(iii) By heating a mixture of alumina and carbon in a current of dry chlorine.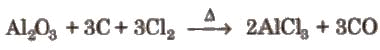
Properties
(i) AlC13 fumes in moist air due to hydrolysis.
AlC13 + 3H2O → Al(OH)3 + 3HCI
(ii) It behaves as Lewis acid.
Uses
It is used as a catalyst in Friedel-Craft reaction and as a mordant dye.
2. Aluminium Oxide or Alumina [AI2O3]
It is the most stable compound of aluminium and occurs in nature as colourless corundum and several coloured oxides, (it present in combination with different metal oxides) like ruby (red), topaz (yellow), sapphire (blue), and emerald (green), which are used as precious stones (gems).
Alum:
The term alum is given to double sulphates of the type X2SO4 * Y2(SO4)3 * 24H2O where, X represents a monovalent cation such as Na+, K+ and NH+4, while Y is a trivalent cation such a Al3,Cr3+, Fe3+ and Co3+(Li+ does not form alum).
Potash Alum
Some important alums are:
(i) Potash alum K2SO4 * Al2(SO4)3 * 24H2O
(ii) Sodium alum Na2SO4 * A12(SO4)3. 24H2O
(iii) Ammonium alum (NH4)2SO4 * AI2(SO4)3 24H2O
(iv) Ferric alum (NH4)2SO4 * Fe2(SO4)3 24H2O
Potash alum is prepared in the laboratory by mixing hot equimolar quantities of K2SO4 and Al2(SO4)3. The resulting solution on concentration and crystallisation gives potash alum.
Note:
(i) A mixture of Al powder NH4NO3 is called ammonal and is lUed in bombs.
(ii) Al is the chief constituent of silver paints.
(iii) A12(SO4)3 1.8 used for making fireproof clothes.
3. Thermite
A mixture of aluminium powder and ferric oxide in the ratio 1: 3.
4. Aluminium Sulphate, Al2(SO4)3
It is used for obtaining H2S in pure form and for making fireproof clothes.
5. Alloys of Aluminium
Alums
The term alum is given to double sulphates of the type X2SO4, Y2(SO4)3. 24H2O where X represents a monovalent cation such as Na+,K+ and NH Rb+, Cs+, Ag+ while Y is a trivalent cation such a Al3+, Cr3+ and Fe3+, Co3+, Ga3+, V3+, Ti3+. (Li+ is too small to be accommodated in the lattice).
General formula or
or 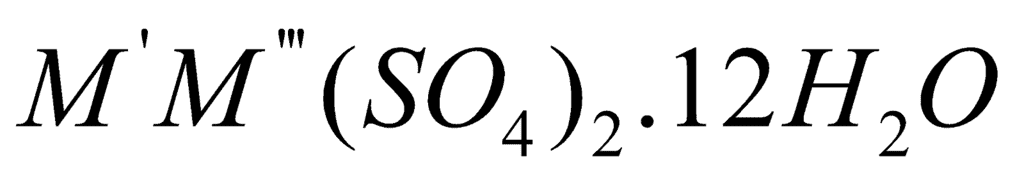
Some important alums are:
1. Potash alum K2SO4. Al2(SO4)3. 24H2O
2. Sodium alum Na2SO4. Al2(SO4)3. 24H2O
3. Ammonium alum (NH4)2SO4. Al2(SO4)3. 24H2O
4. Ferric alum (NH4)2SO4. Fe2(SO4)3. 24H2O
5. Chrome alum K2SO4. Cr2(SO4)3. 24H2O
Pseudo Alums
1. FeSO4.Al2(SO4)3.24H2O → Ferrous aluminium pseudo alum
2. MnSO4.Al2(SO4)3.24H2O → Manganese aluminium pseudo alum
Properties
- Potash alum is a white crystalline compound.
- The aqueous solution of all alums is acidic due to hydrolysis of Al2(SO4)3, Cr2(SO4)3 or Fe2(SO4)3 as given below
- On heating all alums lose water of crystallization
and swell up. The anhydrous alum is known as burnt alum. - Ionisation of aqueous solution of a double salt is as
Uses
- In purification of water.
- For sizing of paper.
- As a styptic to stop bleeding.
- As a mordant in dyeing and tanning of leather.
|
75 videos|278 docs|78 tests
|
FAQs on Group-13 Elements: Boron Family - Chemistry Class 12 - NEET
| 1. What are the physical properties of Group 13 elements? |  |
| 2. What are the trends in physical properties of Group 13 elements? |  |
| 3. What are the chemical properties of Group 13 elements? |  |
| 4. What is the anomalous behavior of Boron? |  |
| 5. What is the diagonal relationship between Boron and Silicon? |  |






















