Group-14 Elements: Carbon Family | Chemistry Class 12 - NEET PDF Download
What are Group 14 Elements?
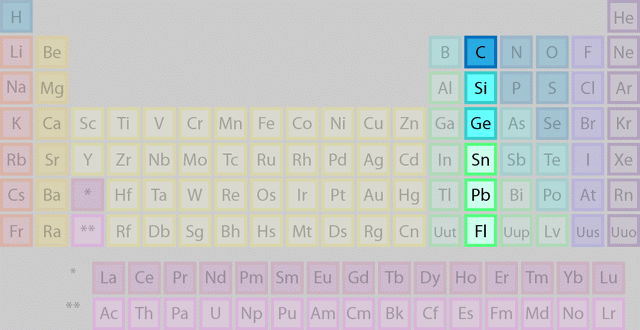
The group 14 elements are the second group in the p-block of the periodic table. It is also called the carbon group. The members of this group are:
- Carbon (C)
- Silicon (Si)
- Germanium (Ge)
- Tin (Sn)
- Lead (Pb)
- Flerovium (Fl)
General Physical Properties of Group 14 Elements
Electronic configuration:
Their valence shell electronic configuration is ns2 np2.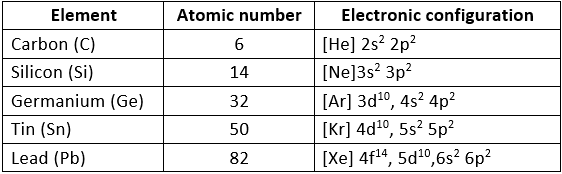
Metallic character:
C and Si are non-metals, Ge is a metalloid and Sn and Pb are metals.Appearance:
C is black. Si is light-brown, Ge is greyish, Sn and Pb are silvery white.Density:
Density increases with an increase in atomic number due to an increase in mass per unit volume down the group.Question for Group-14 Elements: Carbon FamilyTry yourself:Which of the following group 14 elements is a metal?View SolutionMelting points and Boiling points:
The melting points and boiling points decrease from carbon to lead but carbon and silicon have very high melting and boiling points due to their giant structure.Oxidation state:
They exhibit +2 and +4 oxidation state.
The compounds of Pb in the +4 oxidation state are powerful oxidizing agents since the +2 oxidation state of Pb is more stable due to the inert pair effect.
The compounds in the +2 oxidation state are ionic in nature and the + 4 oxidation state are covalent in nature (According to Fajan’s rule).Question for Group-14 Elements: Carbon FamilyTry yourself:What is the fajan’s rule about?View SolutionIonisation enthalpy:
It decreases from C to Sn. For Pb, it is slightly higher than Sn.Electronegativity values:
The value decreases from C to Pb but not in a regular manner probably due to the filling of d-orbitals III and Sn and f- orbitals in Pb.Catenation:
The greater the strength of the element-element bond, the greater is the strength of catenation. C >> Si > Ge = Sn > Pb (catenation).Allotropy:
All the elements of this group except Pb exhibit allotropy.Valency:
All elements exhibit tetra valency. In the case of carbon, 406 kJ mol-1 of energy is required for the promotion of 2s – electron to 2p. The formation of two extra bonds provides this energy.Atomic and ionic radii:
Both increase from C to Pb.- Multiple bonding Carbon forms pπ – pπ bonds with itself and with S, N and O. Other elements show a negligible tendency of this type due to their large size. Others form dπ – pπ multiple bonds.
Note: In cold countries, white tin changes to grey tin and results in a decrease in density. This is called tin disease or tin plague.
Chemical Properties of Group 14 Elements
1. Hydrides:
- All members of the group form covalent hydrides. Their number and ease of formation decrease down the group.
- Hydrides of carbon are called hydrocarbons (alkanes, alkenes or alkynes).
- Hydrides of Si and Ge are known as silanes and germanes.
- The only hydrides of Sn and Pb are SnH4 (stannane) and PbH4 (plumbane).
- Their thermal stability decrease down the group.
- Their reducing character increases down the group.
2. Halides:
- All the elements give tetrahedral and covalent halides of the type MX4 except PbBr4, and PbI4.
- Thermal stability: CX4 > SiX4 > GeX4 > SnX4 > PbX4
- Order of thermal stability with common metals: MF4 > MCl4 > MBr4 > MI4
- Except CX4 other tetrahalides can be hydrolysed due to the presence of vacant d-orbitals.
SiX4 + 2H2O → SiO2 + 4HX. - Ease of hydrolysis: SiX4 > GeX4 > SnX4 > PbX4
- Except for C, other elements form dihalides of the type MX2 which are all ionic and have higher melting points and boiling points, e.g., SnCl2 is a solid whereas SnCl4 is a liquid at room temperature.
Note:
SnCl2 . 5H2O is called bitter of tin and is used as a mordant in dyeing.
3. Oxides:
- They form two types of oxides. mono-oxides of the type MO and dioxides of the type MO2.
Example: CO (neutral) and SiO, GeO, SnO, PbO(all basic)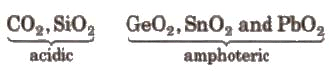
- CO2 is linear gas at ordinary temperature. Solid CO2 is known as dry ice or drikold.
- SiO2 is solid with a three-dimensional network in which Si is bonded to four oxygen atoms tetrahedrally and covalently. A mass of hydrated silica (SiO2) formed from skeletons of minute plants, known as diatoms, is called kieselguhr. It is a highly porous material and is used in the manufacture of dynamite.Question for Group-14 Elements: Carbon FamilyTry yourself:How many types of oxides do Carbon family form?View Solution
Carbon
- The amount of carbon present in the earth’s atmosphere and its crust is very less. There is only 0.02% of carbon in the earth’s crust. This carbon exists as minerals like coal, carbonates and hydrogen carbonates etc. 0.03 % of the carbon exists in the atmosphere of the earth as carbon dioxide.
- Carbon is of utmost importance for our existence and it finds extensive usage in chemistry.
- Because of its indisputable importance, chemistry has been divided into two branches:
(a) Organic Chemistry: This deals with the various compounds containing carbon.
(b) Inorganic Chemistry: This branch deals with compounds that do not have any carbon content. - Free states (diamond. graphite, coal etc.) and combined states (oxides, carbonates, hydrocarbons etc.)
Anomalous Behaviour of Carbon
Anomalous Behaviour of Carbon
- Since most of the first members of a group have peculiar characteristics and properties. On similar grounds, even carbon behaves differently than the other members of the group. These properties of carbon are very unique.
- We can attribute this behaviour to carbon mainly due to:
(i) Small size of the atom
(ii) High electronegativity
(iii) High ionization enthalpy
(iv) Unavailability of d-orbital’s
Unique Properties of Carbon
1. Small Size of Carbon
- Carbon derives a lot of its properties from its small size.
- The compounds that carbon forms are highly stable and this is also because of their small size. Due to its small size, the nucleus effectively holds on to the bonded and non-bonded electrons.
2. Tetravalency of Carbon
- Carbon exhibits tetravalency. It means it can share four electrons to complete its octet. Thus, we know it bonds to four different monovalent atoms.
- Carbon forms a large variety of compounds with oxygen, nitrogen, hydrogen, halogens. This results in a different set of compounds that have distinctive characteristics and properties.
- Carbon has the availability of only s and p orbitals. Therefore, it can hold only four pairs of electrons in its valence shell. Thus, we can restrict the covalence to four. However, the other elements in the group can easily grow their covalence due to the availability of d-orbitals.
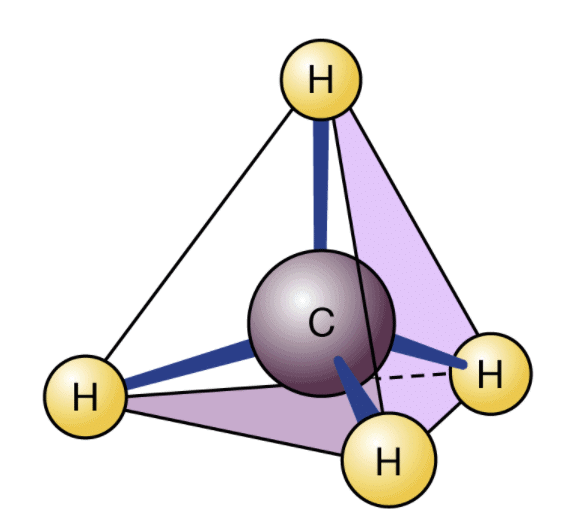
3. Catenation
- One of the unique properties of Carbon is its ability to form long carbon chains. It implies that carbon attaches with other carbon atoms to form long carbon chains. This property is known as catenation. Sometimes, this chain could be as big as to have a total of 70-80 carbons.
- This gives rise to a variety of complex compounds. Some of the compounds have a straight carbon chain while some others have branched carbon chain or rings.
- The carbon compounds having only a single bond are saturated hydrocarbons. On the other hand, the compounds with the double or triple bond are unsaturated hydrocarbons.
- As we move down the group, the size of the elements increases. This results in decreasing electronegativity. Thus, the propensity to show catenation also decreases. This can be clearly observed from bond enthalpy values.
- The catenation order: C >> Si > Ge >> Sn.
4. Electronegativity
- Additionally, carbon has an extraordinary capacity to shape pp – pp multiple bonds with itself and with different molecules. This can also be related to its smaller size and high electronegativity. Some of the examples would include C = C, C° C, C = O, C = S and C° N.
- As a matter of fact, the heavier elements don’t shape pp – pp bonds. This is mainly because of the reason that their nuclear orbitals are too vast and diffused to have viable overlapping.
Example: Lead does not indicate catenation.
Q. Give some practical uses of carbon.
Ans. There are many important uses of carbon. Some of them are:
- We use impure carbon in the form of charcoal (from wood) and coke (from coal) in metal smelting.
- Graphite is a common use in pencils. We also use graphite to make brushes in electric motors and in furnace linings.
- Activated charcoal finds its usage in purification and filtration in respirators and kitchen extractor hoods.
- Industrial diamonds are a common tool for cutting rocks and drilling.
Allotropic Forms of Carbon
The crystalline forms include:
1. Diamond:
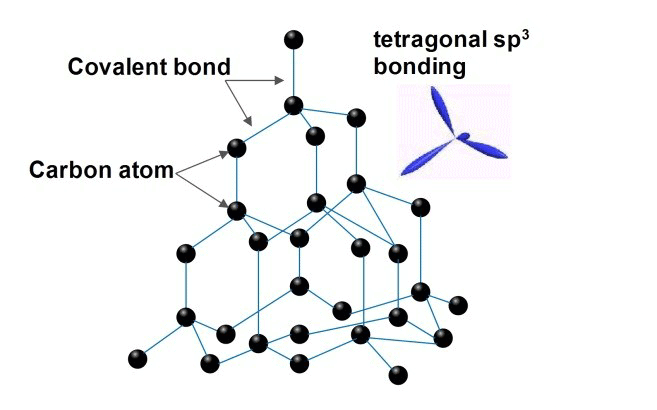
- It is the hardest and has a three-dimensional polymeric structure in which the hybridization of C is sp3.
- It is a covalent solid.
- Melting point = 3650 °C
- Density = 3.51 g/cm3
- Bad Conductor of heat and electricity.
2. Graphite:
- It is dark grey having hexagonal plates, hybridization of each C is sp2.
- It is a good conductor of heat and electricity due to the presence of free electrons.
- It was also known as the black lead.
- It is a very good lubricant.
- Aqua dag: Suspensions of graphite in water.
- Oil dag: Suspension of graphite in oil lubricants.
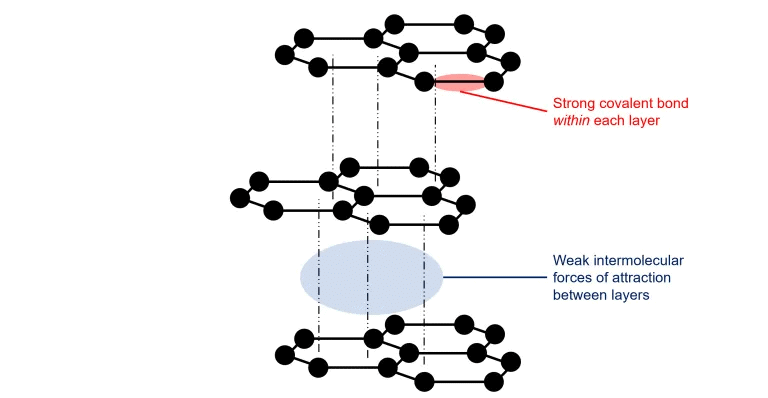 Structure of Graphite
Structure of Graphite
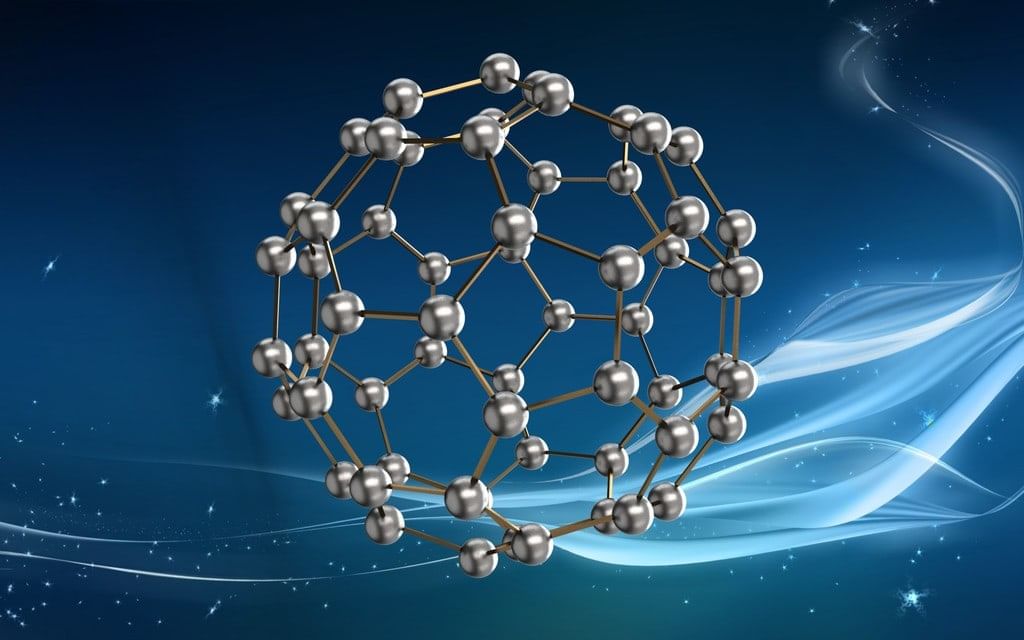
3. Fullerenes:
- These are the only pure form of carbon.
- C60 molecule contains 12 five-membered rings and 20 six-membered rings.
- The five-membered rings are connected to six-membered rings while six-membered rings are connected to both five and six-membered rings.
- These are used in microscopic ball bearings, lightweight batteries, in the synthesis of new plastics and new drugs.
 Question for Group-14 Elements: Carbon FamilyTry yourself:Which of the following allotropes of carbon are hard in nature?View Solution
Question for Group-14 Elements: Carbon FamilyTry yourself:Which of the following allotropes of carbon are hard in nature?View Solution
Amorphous forms of carbon are:
- Coal: The different forms of coal are peat (60 % C), lignite (70 % C), Bituminous (78 % C), Semi Bituminous (83 % C) and anthracite (90 % C). Bituminous is the most common variety of coal.
- Coke is obtained by the destructive distillation of coal.
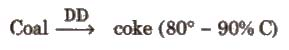
- Charcoal or wood charcoal: It is obtained by heating wood strongly in absence of air. When heated with steam, it becomes more activated. It is used to remove colouring matters and odoriferous gases.
- Bone black or animal charcoal It is obtained by destructive distillation of bones in iron retort. By-products are bone oil or pyridine. It is used as an adsorbent. On burning, it gives bone ash which is calcium phosphate and used in the manufacture of phosphorous and phosphoric acid.
- Lamp-black It is obtained by burning vegetable oils in a limited supply of air. It is used in the manufacture of printing ink, black paint, varnish and carbon paper.
- Carbon-black It is obtained by burning natural gas in a limited supply of air. It is added to a rubber mixture for making automobile tyres.Question for Group-14 Elements: Carbon FamilyTry yourself:Which of the following is an amorphous allotrope of carbon?View Solution
Gaseous Forms of Carbon
Coal Gas:
Preparation: By destructive distillation of coal.
Composition: H2 = 45 – 55%, N2 = 2 – 12%, CH4 = 25 – 35%, CO2 = 0 – 3%, CO = 4 – 11%, O2 = 1 – 1.5%, Ethylene, acetylene, benzene, etc. = 3 – 5 %
Uses: It is used as an illuminant, as fuel and to provide an inert atmosphere in the metallurgical processes.Natural Gas:
It is found along with petroleum below the surface of the earth.
Composition: CH4 = 60 – 80 %, Higher hydrocarbons = 2 – 12%, C2H6 = 5 – 10 %, C3H8 = 3 – 18 %
Uses: It is used as a fuel. Its partial combustion yields carbon black (reinforcing agent for rubber).Oil Gas:
Preparation: Uses: It is used as fuel in laboratories in Bunsen burners.
Uses: It is used as fuel in laboratories in Bunsen burners.Wood-Gas:
Preparation: Destructive distillation of wood gives wood gas (CH4, C2H6 H2)
Uses: It is used as fuel.Liquified Petroleum Gas (LPG):
Composition: n-butane + Iso-butane
Uses: It is used as domestic fuel.Carbon Monoxide (CO):
Preparation:
It is manufactured in the form of water and produces gas.Properties:
(i) It is a colourless, odourless and almost water-insoluble gas.
(ii) It is a powerful reducing agent.
(iii) CO is used in the extraction of many metals from their oxide ores.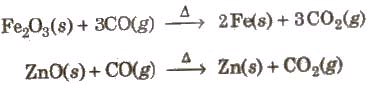
Carbon Dioxide (CO2):
Preparation:
(i)
(ii)
(iii)
Properties:
(i) It is a colourless and odourless gas.
(ii) With water, it forms carbonic acid.
(iii) Photosynthesis
Compounds of Silicon
Silicates
Silicates are metal derivatives of silicic acid, H2SiO3 and can be obtained by fusing metal oxides or metal carbonates with sand. The basic structural unit of silicates is SiO44-.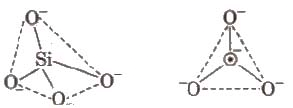
Mica (abrak) is naturally occurring aluminium silicate [KH2AI3(SiO4]3 or KAI3Si3O10(OH)2.Silicones
The linear, cyclic or cross-linked polymeric compounds containing (R2SiO) as a repeating unit, are known as silicones. They are manufactured from alkyl-substituted chlorosilanes.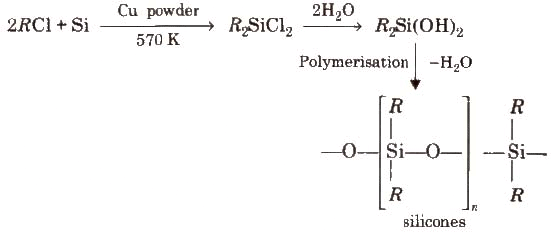 Silicones are chemically inert, water repellent, heat resistant, good electrical insulators. These are used as lubricants (vaseline), insulators etc
Silicones are chemically inert, water repellent, heat resistant, good electrical insulators. These are used as lubricants (vaseline), insulators etc
It is the second hardest material known and has the formula SiC (silicon carbide). It is used as a high-temperature semiconductor, in transistor diode rectifiers.Carborundum
It is a transparent or translucent amorphous substance obtained by fusion of sodium carbonate (or sodium sulphate), calcium carbonate and sand (silica). It is not truly solid, so its melting point is not sharp. The general formula of glass is Na2O.CaO.6SiO2Glass
Coloured glasses are obtained by adding a certain substance to the molten mass.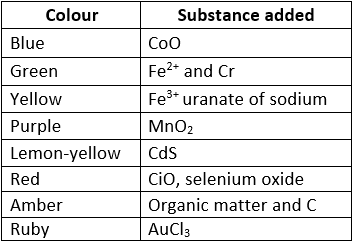 Different Varieties of Glass:
Different Varieties of Glass: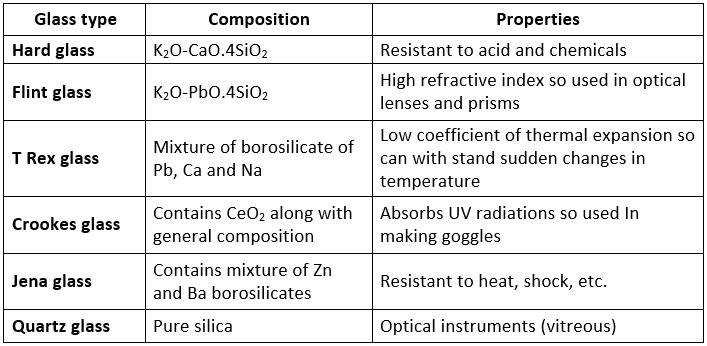 Glass is attacked by HF. This property is used in the etching of glass.
Glass is attacked by HF. This property is used in the etching of glass.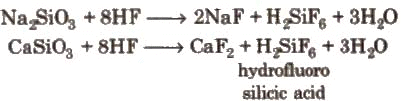 Question for Group-14 Elements: Carbon FamilyTry yourself:The cross-linked polymer compounds containing silicone which are linear and the cyclic are called ___________View Solution
Question for Group-14 Elements: Carbon FamilyTry yourself:The cross-linked polymer compounds containing silicone which are linear and the cyclic are called ___________View Solution
Compounds of Lead
Chrome yellow (PbCrO4):
It is prepared by adding potassium chromate to lead chromate and is used as a yellow pigment under the name chrome yellow. On treating with alkali, it gives basic lead chromate or chrome red, PbCrO4.PbO.Basic lead carbonate, Pb(OH)2.2PbCO3
It is also known as white lead and is prepared by adding sodium carbonate solution to any lead salt.
3Pb(NO3)2 + 3Na2CO3 + H2O → Pb(OH)2.2PbCO3 + 6NaNO3 + CO2
It is used as white paint. The disadvantage of using white lead in paints is that it turns black by the action of H2S of the atmosphere.
Note:
Lead poisoning is called plumbosolvency which increases in the excess of nitrates, organic acids and ammonium salts.
Unique Properties of Carbon
1) The Small Size of Carbon:- Carbon derives a lot of its properties from its small size. The compounds that carbon forms are highly stable and this is also because of its small size. Due to its small size, the nucleus effectively holds on to the bonded and non-bonded electrons.
- Hence in short, tetravalency, small size and property of catenation make carbon different from other elements and so we have the whole branch of chemistry dedicated to the study of this kind of compound.
2) Tetravalency of Carbon:
- Carbon exhibits tetravalency. It means it can share four electrons to complete its octet. Thus, we know it bonds to four different monovalent atoms. Carbon forms a large variety of compounds with oxygen, nitrogen, hydrogen, halogens. This results in a different set of compounds which have distinctive characteristics and properties.
- Carbon has the availability of only s and p orbitals. Therefore, it can hold only four pairs of electrons in its valence shell. Thus, we can restrict the covalence to four. However, the other elements in the group can easily grow their covalence due to the availability of d-orbitals.
3) Catenation:
- One of the unique properties of Carbon is its ability to form long carbon chains. It implies that carbon attaches with other carbon atoms to form long carbon chains. This property is what we call as catenation. Sometimes, this chain could be as big as to have a total of 70-80 carbons. This gives rise to a variety of complex compounds. Some of the compounds have a straight carbon chain while some others have branched carbon chain or rings.
- The carbon compounds having only single bond are the saturated hydrocarbons. On the other hand, the compounds with double or triple bond are the unsaturated hydrocarbons.
- As we move down the group, the size of the elements increases. This results in a decreasing electronegativity. Thus, the propensity to show catenation also decreases. This can be clearly observed from bond enthalpy values. The catenation order is C >> Si > Ge >> Sn.
4) Electronegativity:
- Additionally, carbon has an extraordinary capacity to shape pp – pp multiple bonds with itself and with different molecules. This can also be related to its smaller size and high electronegativity. Some of the examples would include C = C, C° C, C = O, C = S and C° N.
- As a matter of fact, the heavier elements don’t shape pp – pp bonds. This is mainly because of the reason that their nuclear orbitals are too vast and diffused to have viable overlapping. For example, lead does not indicate catenation.
Solved Example:
Q: Give some practical uses of carbon.
Ans: There are many important uses of carbon. Some of them are:
- We use impure carbon in the form of charcoal (from wood) and coke (from coal) in metal smelting.
- Graphite is a common use in pencils. We also use graphite to make brushes in electric motors and in furnace linings.
- Activated charcoal finds its usage in purification and filtration in respirators and kitchen extractor hoods.
- Industrial diamonds are a common tool for cutting rocks and drilling.
|
75 videos|278 docs|78 tests
|
FAQs on Group-14 Elements: Carbon Family - Chemistry Class 12 - NEET
| 1. What are the general physical properties of Group 14 elements? |  |
| 2. What are the unique properties of carbon compared to other Group 14 elements? |  |
| 3. What are the allotropic forms of carbon and how do they differ from each other? |  |
| 4. What are the compounds of silicon and how do they differ from those of carbon? |  |
| 5. What is the anomalous behavior of carbon and how does it contribute to its versatility in forming compounds? |  |

















