NEET Exam > NEET Notes > Chemistry Class 12 > Group-16 Elements: Oxygen Family
Group-16 Elements: Oxygen Family | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| What are Group 16 Elements? |

|
| General Physical Properties of Group 16 Elements |

|
| Chemical Properties of Group 16 Elements: |

|
| Oxygen and its Compounds: |

|
| Compounds of Sulphur |

|
What are Group 16 Elements?
The group 16 elements of the modern periodic table consist of 5 elements oxygen, sulphur, selenium, tellurium and polonium. The elements in this group are also known as the chalcogens or the ore-forming elements because many elements can be extracted from sulphide or oxide ores.
The name sulphur has been derived from Sanskrit word ‘Sulvezi’ meaning ‘killer of copper’.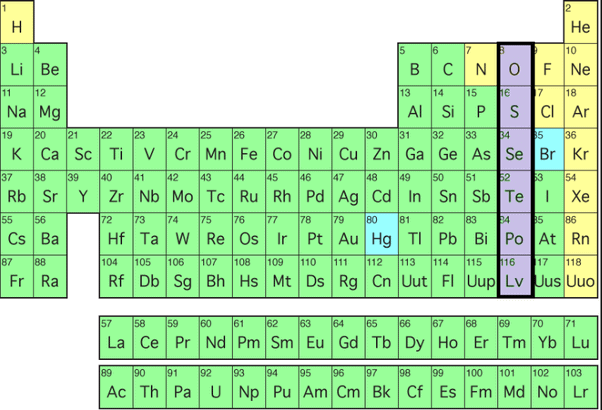
General Physical Properties of Group 16 Elements
Electronic configuration:
Their valence shell electronic configuration is ns2np4.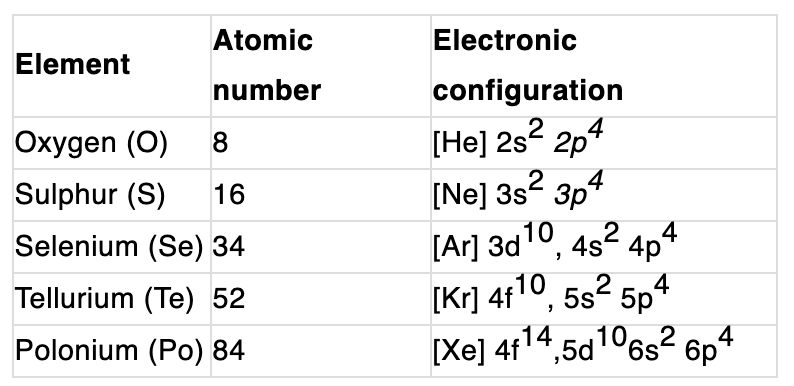
Metallic and non-metallic character:

Abundance:
O > S > Se > Te > PoDensity:
It increases down the group regularly,Melting point and boiling point:
Both show a regular increase down the group due to an increase in molecular weight and Van der Waals’ forces of attraction.Oxidation state:
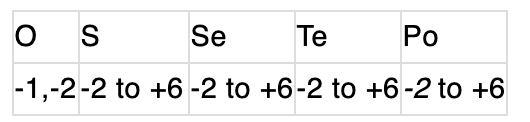 In OF2, the oxidation state of oxygen is +2.
In OF2, the oxidation state of oxygen is +2.Ionisation energy:
They possess a large amount of ionisation energy which decreases gradually from O to Po due to an increase in the size of atoms and an increase in the screening effect.Electron affinity:
They have high electron affinity which decreases from O to Po. As the size of the atom increases, the extra added electron feels lesser attraction by the nucleus and hence, electron affinity decreases.Electronegativity:
It decreases down the group due to a decrease in effective nuclear charge down the group.Catenation:
Group 16 elements follow the order as shown below: S-S > Se-Se > O-O > Te-TeAtomicity:
Oxygen is diatomic, sulphur and selenium are octatomic with a puckered ring structure.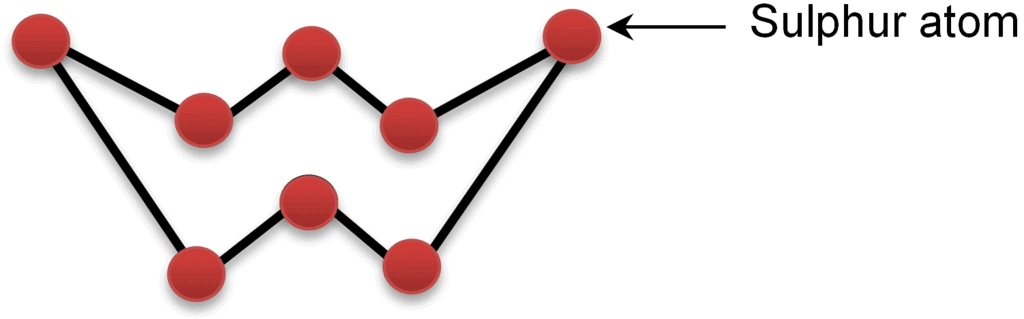
Allotropy:
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements.
- Oxygen – Dioxygen (O2) and ozone (O3)
- Sulphur – Rhombic (01′ a) sulphur S8
- Monoclinic (or β) sulphur, S8(most stable), plastic sulphurAtomic radii and ionic radii:
They increase regularly from O to Po.
Chemical Properties of Group 16 Elements:
Hydrides:
All these elements form stable hydrides of the type H2E. (Where. E = O, S, Se, Te and Po).
2H2 + O2 ⇔ 2H2O
FeS + H2SO4 → H2S + FeSO4
H2O is a liquid due to hydrogen bonding, while others are colourless gases with an unpleasant smell.
[Down the group acidic character increases from H2O to H2Se. All the hydrides except water possess reducing property and this character increases from H2 S to H2Te].Halides:
The stability of the halides decreases in the order: F– > Cl– > Br– > I–Amongst hexahalides, hexafluorides are the only stable halides. All hexafluorides are gaseous in nature. SF6 is exceptionally stable for steric reasons.
SF4 is a gas, SeF4 is a liquid and TeF4 is solid. These fluorides have sp3 d hybridization and see-saw geometry. They behave Lewis acid as well as Lewis base e.g.,
SF4 + BF3 → SF4 → BF3
SeF4 + 2F– → [SeF6]2-
The well known mono halides are dimeric in nature. Example are S2F2, S2Cl2, S2Br2, Se2Cl2 and Se2Br2. These dimeric halides undergo disproportion as given below:
2 SeCl2 → SeCl4 + SeOxides:
They form AO2 and AO3 type oxides. Their acidic nature follow the order: SO2 > SeO2 > TeO2 > PoO2 and SO3 > SeO3 > TeO3
Ozone is considered as oxides of oxygen.
SO2 is a gas having sps -hybridisation and V-shape.
SO3 is a gas that is sp2-hybridised and planar in nature.
SeO2 is a volatile solid consist of non-planar infinite chains.
SeO3 has a tetrameric cyclic structure in solid-state. SO2 and SO3 are the anhydrides of sulphurous (H2SO3) and sulphuric acid (H2SO4) respectively.Question for Group-16 Elements: Oxygen FamilyTry yourself:Which of the following can classified as an amphoteric oxide?View Solution
Note: In photocopying (xerox) machines, Se acts as a photoconductor.
Oxygen and its Compounds:
Dioxygen:
Priestley and Scheele prepared oxygen by heating suitable oxygen compounds.
Preparation: By action of heat on oxygen-rich compounds.
(i) From oxides: (ii) From peroxides and other oxides:
(ii) From peroxides and other oxides: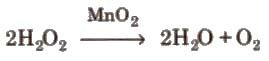
(iii) From certain compounds: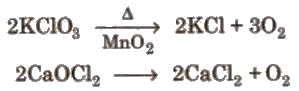
Physical properties: It is colourless, odourless, tasteless, slightly heavier than air and sparingly soluble in water.
Chemical properties: On heating, it combines directly with metals and non-metals, e.g.,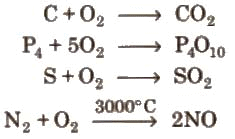
2Mg + O2 → 2MgO
4Na + O2 → 2 Na2O → Na2O2
Combination with O2 is accelerated by using catalyst. Platinum is particularly an active catalyst.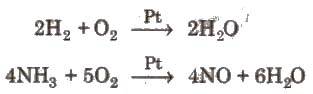
Uses: It is used in welding and cutting oxy-hydrogen or oxy-acetylene torch and in the iron and steel industry to increase the content of blast in the Bessemer and open-hearth process. It is also used for life support systems e.g., in hospitals, for divers, miners and mountaineers.
Tests:
1. With NO it gives reddish-brown fumes of NO2.
2. It is adsorbed by alkaline pyrogallol.Question for Group-16 Elements: Oxygen FamilyTry yourself:Which of the following in air, forms compounds readily?View SolutionOzone (O3)
Preparation: Bypassing silent electric discharge through cold, dry oxygen in ozonised.
Lab method: 3O2 ⇔ 2O3; + 284.3 kJ
Physical properties: It is pale blue gas with a characteristic strong smell. It is slightly soluble in water.
Chemical reactions:
1.Decomposition: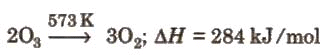 2. Oxidising action:
2. Oxidising action:3. It acts as a powerful oxidizing agent. It liberates iodine from neutral KI solution and the liberated I2 turns starch paper blue.
2KI + H2 + O3 → 2KOH + I2 + O2
I2 + Starch → Blue colour
Uses: It is used
1. as a germicide and disinfectant for sterilizing water.
2. as a bleaching agent for oils, ivory wax and delicate fibres.
3. for detecting ‘the position of the double bond in unsaturated compounds.
4. in destroying odours coming from the cold storage room, slaughterhouses and kitchen of hotels.
Compounds of Sulphur
Sulphur Dioxide (SO2)
Method of preparation:
(i) By heating sulphur in the air(ii) Roasting iron pyrites in excess of air
(iii) Lab method
Physical Properties: SO2 is a colourless gas with a pungent smell and is highly soluble in water.
Chemical reactions: It turns lime water milky due to the formation of calcium bisulphite. However, in excess of SO2 milkiness disappears due to the formation of calcium bisulphite.
Ca(OH)2 + S02 → CaS03 + H20 (milkiness)
CaSO3 + S02 + H20 → Ca(HSO3)2 (soluble)
2NaOH + S02 → Na2SO3 + H20
Na2SO3 + H20 + S02 → 2NaHSO3
S02(g) + CL2(g) → S02Cl2(l)
2S02(g) + 02(g) → 2SO3(g)
Reducing agent
2Fe3+ + S02 + 2H20 → 2Fe2+ + SO42- + 4H+
5S02 + 2MnO42- + 2H20 → 5SO42- + 4H+ + 2Mn2+
When H2S gas is passed through a saturated solution of SO2 till its smell disappears, it turns in a milky solution, the Wacken roder’s liquid. When H2S is passed through H2SO4 the reaction is called Wacken roder’s reaction.Oxoacids of Sulphur:
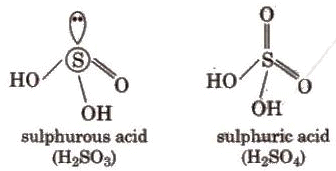
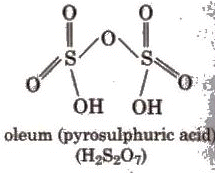
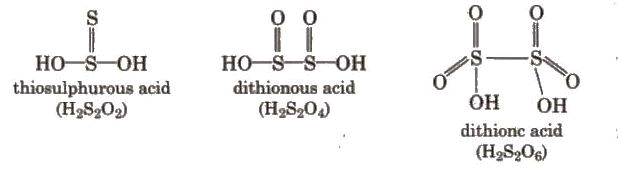
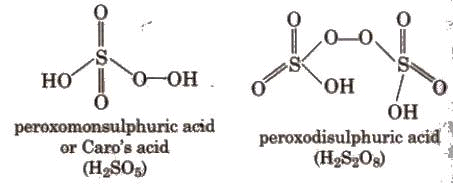
Sulphuric Acid (H2SO4)
Sulphuric acid is one of the most important industrial chemicals worldwide. It is called the king of chemicals. It is manufactured by the lead chamber process or contact process. The contact process involves three steps:
(i) Burning of sulphur or sulphur ores in air to generate SO2.
(ii) Conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5).
(iii) Absorption of SO3 in H2SO4 to give oleum (H2S2O7) which upon hydrolysis gives H2SO4.
Properties:
1. Sulphuric acid is a colourless, dense, oily liquid.
MX + H2SO4 → 2HX + M2SO4
2. Concentrated sulphuric acid is a strong dehydrating agent. The burning sensation of concentrated H2SO4 on skin.
The burning sensation of concentrated H2SO4 on skin.
3. Hot concentrated sulphuric acid is a moderately strong oxidising agent. In this respect, it is intermediate between phosphoric acid and nitric acid.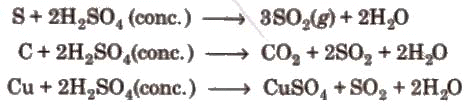
Uses: It is used in petroleum refining, in pigments paints and in detergents manufacturing.Hypo:
It is chemically sodium thiosulphate pentahydrate, Na2S2O3.5H2O. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. It is also called sodium hyposulfite or "hypo".
Preparation:
1. It is prepared by boiling sodium sulphite solution with flowers of sulphur and stirring till the alkaline reaction has disappeared.
Na2SO3 + S → Na2S2O3
2. It is also prepared by spring’s reaction.
Na2S + Na2SO3 + I2 → Na2S2O3 +2NaI
Properties:
(i). It is a colourless, crystalline and efflorescent substance.
(ii). It gives white ppt with a dilute solution of AgNO3 which quickly changes into black due to the formation of Ag2S.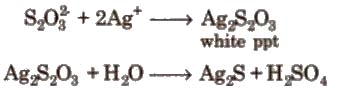
Uses:
1. Due to its property of dissolving silver halide, it is used in photography for fixing under the name hypo.
2Na2S2O3 + AgBr → Na3 [ Ag(S2O3)2] + NaBr
2. During bleaching, it is used as an antichlor.
Na2S2O3 + CI2 + H2O → Na2SO4 + S + 2HCI
3. It is used to remove iodine stain, for volumetric estimation of iodine and in medicines.
Question for Group-16 Elements: Oxygen FamilyTry yourself:Which of the following is the most popular oxoacid of sulfur?
View Solution
The document Group-16 Elements: Oxygen Family | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|278 docs|78 tests
|
FAQs on Group-16 Elements: Oxygen Family - Chemistry Class 12 - NEET
| 1. What are the general physical properties of Group 16 elements? |  |
Ans. The general physical properties of Group 16 elements include being non-metals, having high electronegativity, and existing in various physical states at room temperature. Oxygen and sulfur are the most common elements in this group.
| 2. What are the chemical properties of Group 16 elements? |  |
Ans. The chemical properties of Group 16 elements include having six valence electrons, forming covalent bonds with other elements, and being reactive towards metals. They have a tendency to gain two electrons to achieve a stable electron configuration.
| 3. What are some compounds of oxygen and their significance? |  |
Ans. Oxygen forms various compounds, such as water (H2O) and carbon dioxide (CO2), which are vital for life on Earth. Water is the most abundant compound on the planet and is essential for the survival of all organisms. Carbon dioxide is a greenhouse gas that plays a significant role in Earth's climate.
| 4. What are some compounds of sulfur and their importance? |  |
Ans. Sulfur forms several compounds, including hydrogen sulfide (H2S) and sulfuric acid (H2SO4). Hydrogen sulfide is a toxic gas with a distinct odor, while sulfuric acid is a strong acid used in various industries, such as batteries, fertilizers, and dyes.
| 5. How are the Group 16 elements related to the Oxygen family? |  |
Ans. The Group 16 elements, also known as the chalcogens, are closely related to the Oxygen family. This family includes elements such as oxygen, sulfur, selenium, tellurium, and polonium. They share similar chemical properties and have a tendency to gain electrons to achieve a stable configuration.
Related Searches
















