Important Equations and Definitions: Chemical Reactions and Equations | Science Class 10 PDF Download
Chemical Equations
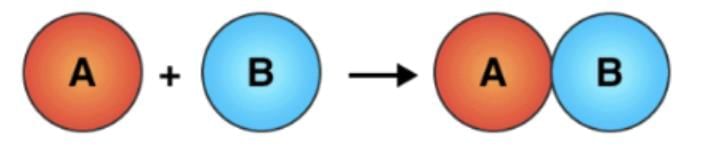
A + B → C + D
In this example, A and B are the reactants, which react to form the products C and D.
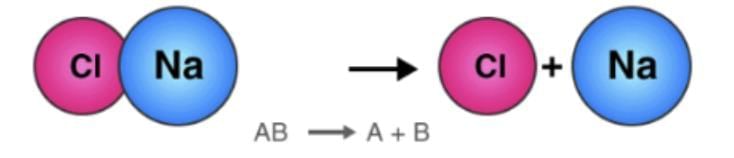
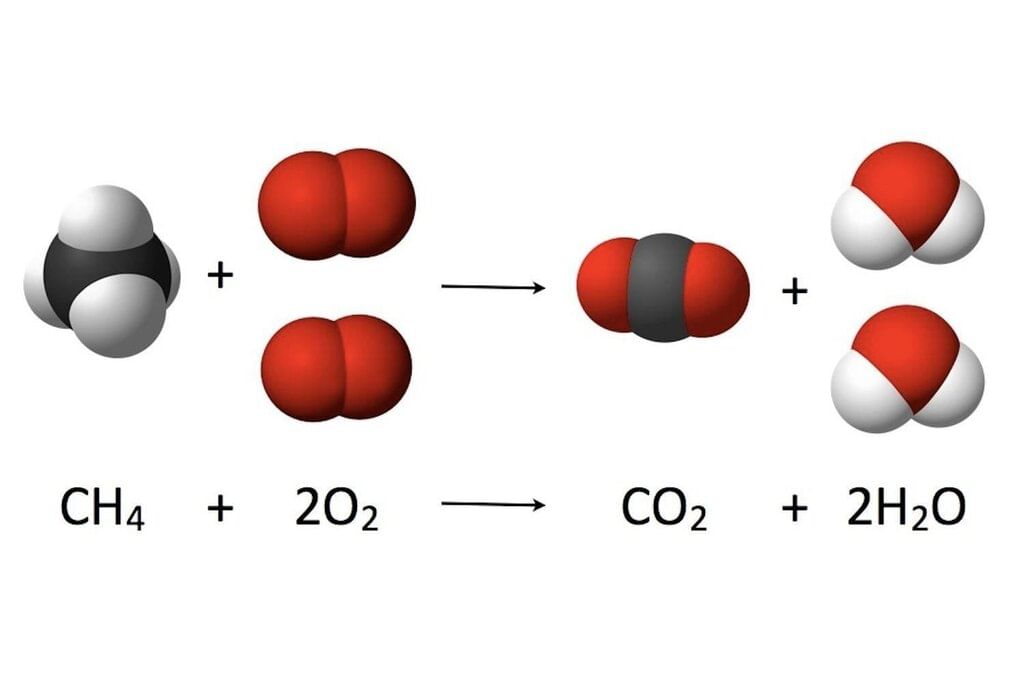 Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Types of Chemical Reactions
1. Combustion Reaction
2Mg + O₂ → 2MgO
A combustion reaction is a reaction with a combustible material with an oxidizer to give an oxidized product.
2. Decomposition Reaction
CaCO₃ → CaO + CO₂
A Decomposition reaction is a reaction in which a single component breaks down into multiple products.
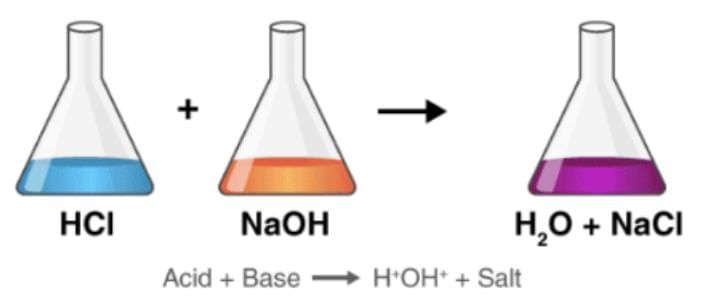
3. Neutralization Reaction
HCl + NaOH → NaCl + H₂O
A Neutralization reaction is the reaction between an acid and a base giving salt and water as the products.
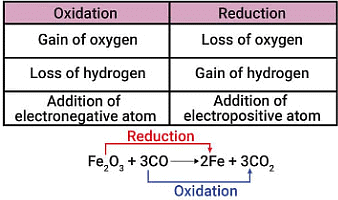
4. Redox Reaction
Zn + 2H⁺ → Zn²⁺ + H₂
A REDuction-OXidation reaction is a reaction in which there is a transfer of electrons between chemical species.
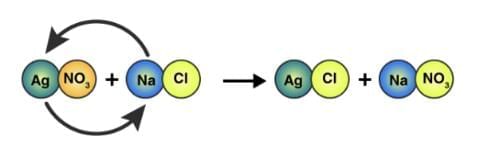
5. Precipitation or Double-Displacement Reaction
AgNO₃ + NaCl → AgCl + NaNO₃
It is a type of displacement reaction in which two compounds react, and their anions and cations switch places forming two new products.
6. Synthesis Reaction
2Na(s) + Cl₂(g) → 2NaCl(s)
A Synthesis reaction is one of the most basic types of reaction wherein multiple simple compounds combine under certain physical conditions giving out a complex product.
Important Points to Remember
- In a chemical change, a new compound is formed but in a physical change, the substance changes its state of existence.
- Atoms or ions or molecules which react to form a new substance are called reactants; the new atoms or molecules formed are products.
- A chemical reaction follows the law of conservation of mass. That is no atom is destroyed or created but only a new product is formed from reactants.
|
80 videos|569 docs|80 tests
|
FAQs on Important Equations and Definitions: Chemical Reactions and Equations - Science Class 10
| 1. What is a chemical equation? |  |
| 2. How do you balance a chemical equation? |  |
| 3. What are the types of chemical reactions? |  |
| 4. What is the difference between a chemical equation and a chemical reaction? |  |
| 5. Why is it important to balance a chemical equation? |  |