Important Equations and Definitions: Carbon and its Compounds | Science Class 10 PDF Download
General Formula of Hydrocarbons
Hydrocarbons are organic compounds consisting of hydrogen and carbon atoms. The general formula for two major classes of hydrocarbons, namely alkanes and alkenes, is as follows:
Alkanes
General formula: CnH2n+2
- Alkanes are saturated hydrocarbons, which means they contain only single covalent bonds between carbon atoms.
- The general formula reflects that for each "n" carbon atoms, there are 2n+2 hydrogen atoms.
Alkenes
General formula: C nH 2n
- Alkenes are unsaturated hydrocarbons, and they contain at least one carbon-carbon double bond.
- The general formula shows that for each "n" carbon atoms, there are 2n hydrogen atoms.
These general formulas help you determine the molecular formula of specific hydrocarbons by substituting the appropriate value of "n." For example, if you have a hydrocarbon with three carbon atoms, the molecular formula for an alkane would be C 3H 8, and for an alkene, it would be C 3H 6.
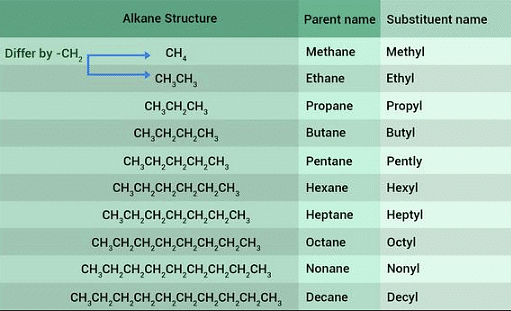
Homologous Series
The homologous series of alkanes has the general formula CnH2n+2. Here are some examples of alkanes within this series:
Functional Group
A functional group is a specific group of atoms within a molecule that determines the chemical properties and reactivity of that molecule.
- It is the part of a molecule that is primarily responsible for its chemical behavior.
- Functional groups are commonly found in organic compounds, and they are crucial in identifying and understanding the characteristics of various organic compounds.
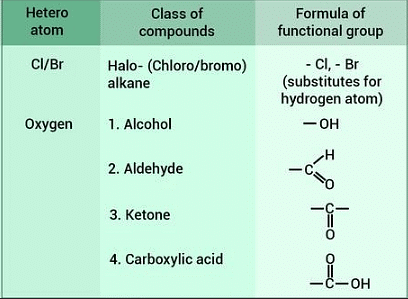
Here are a few examples of some common functional groups that can be introduced to 10th-grade students:
- Hydroxyl Group (-OH): The hydroxyl group is found in alcohols, and it imparts properties like solubility in water and the ability to form hydrogen bonds.
- Carbonyl Group (C=O): The carbonyl group can be found in aldehydes and ketones. Aldehydes have a carbonyl group at the end of the carbon chain, while ketones have it in the middle. It affects the reactivity of these compounds.
- Amino Group (-NH 2): The amino group is found in amines. It makes the compounds basic and able to participate in various chemical reactions.
- Carboxyl Group (-COOH): The carboxyl group is found in carboxylic acids. It gives these compounds acidic properties and allows them to release hydrogen ions (H+) in solution.
- Ester Group (-COO-): The ester group is found in esters, which are common in fragrances and flavorings. It contributes to their characteristic smells and tastes.
- Methyl Group (-CH 3): The methyl group is a simple hydrocarbon group found in various organic compounds. It can influence the physical and chemical properties of the compound it's a part of.
|
80 videos|569 docs|80 tests
|
FAQs on Important Equations and Definitions: Carbon and its Compounds - Science Class 10
| 1. What is the general formula for alkanes, alkenes, and alkynes? |  |
| 2. What are homologous series in organic chemistry? |  |
| 3. What are functional groups and why are they important in organic chemistry? |  |
| 4. How do you identify the functional group in a compound? |  |
| 5. What are some important equations related to carbon and its compounds? |  |

















