UPSC Exam > UPSC Notes > Geography for UPSC CSE > Infographic: Acid Rain
Infographic: Acid Rain | Geography for UPSC CSE PDF Download
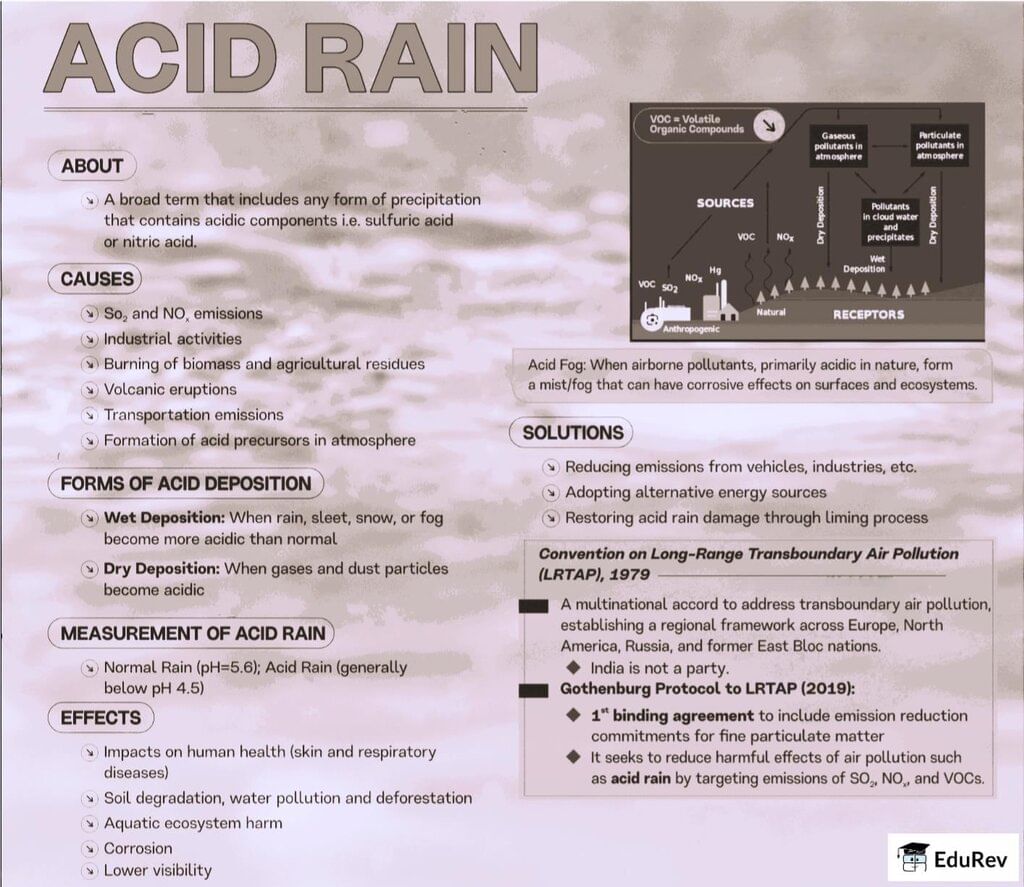
The document Infographic: Acid Rain | Geography for UPSC CSE is a part of the UPSC Course Geography for UPSC CSE.
All you need of UPSC at this link: UPSC
|
263 videos|874 docs|232 tests
|
FAQs on Infographic: Acid Rain - Geography for UPSC CSE
| 1. What is acid rain and how is it formed? |  |
Ans. Acid rain refers to any form of precipitation (rain, snow, sleet, or hail) that has a pH level lower than 5.6, making it acidic. It is formed when sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) are released into the atmosphere, primarily from burning fossil fuels. These gases react with water vapor, oxygen, and other chemicals in the atmosphere to form sulfuric acid (H₂SO₄) and nitric acid (HNO₃), which then fall to the ground as acid rain.
| 2. What are the environmental impacts of acid rain? |  |
Ans. Acid rain has several detrimental effects on the environment. It can lead to the acidification of lakes and streams, harming aquatic life by reducing the availability of essential minerals and increasing toxic metals. It also damages forests by leaching nutrients from the soil and harming tree foliage. Additionally, acid rain can degrade buildings and monuments, especially those made of limestone and marble, leading to increased maintenance costs.
| 3. How does acid rain affect human health? |  |
Ans. While acid rain does not pose a direct health risk to humans, the pollutants that cause it—sulfur dioxide and nitrogen oxides—can contribute to respiratory problems such as asthma and bronchitis. These pollutants can form fine particulate matter that, when inhaled, can exacerbate cardiovascular issues and compromise overall respiratory health.
| 4. What measures can be taken to reduce acid rain? |  |
Ans. To reduce acid rain, several measures can be implemented, including the use of cleaner energy sources such as wind, solar, and hydroelectric power, which produce little to no sulfur dioxide and nitrogen oxides. Regulations and legislation, such as emissions trading systems and the implementation of scrubbers in power plants, can also help minimize emissions. Public awareness campaigns and promoting energy efficiency in homes and industries can further contribute to reducing the pollutants responsible for acid rain.
| 5. What is the significance of the Clean Air Act in relation to acid rain? |  |
Ans. The Clean Air Act is critical in addressing the issue of acid rain as it sets regulations to control air pollution from stationary and mobile sources. Amendments to the Act have specifically targeted reductions in sulfur dioxide and nitrogen oxide emissions, which are the primary contributors to acid rain. The implementation of these regulations has led to significant decreases in emissions, contributing to the overall reduction of acid rain in many regions.
Related Searches
















