NEET Exam > NEET Notes > Chemistry Class 11 > Infographic: Redox Reactions
Infographic: Redox Reactions | Chemistry Class 11 - NEET PDF Download
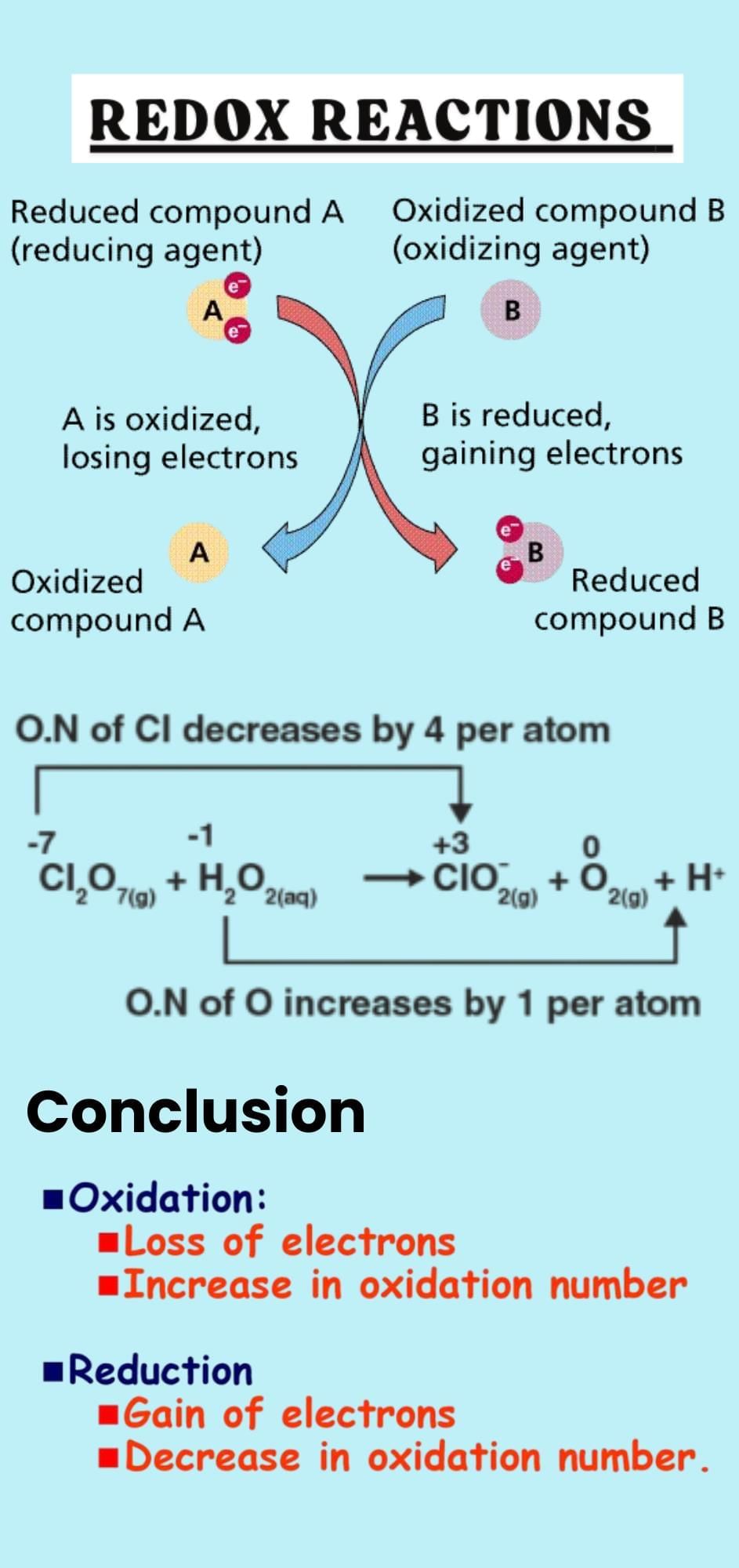
The document Infographic: Redox Reactions | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
114 videos|263 docs|74 tests
|
FAQs on Infographic: Redox Reactions - Chemistry Class 11 - NEET
| 1. What are redox reactions and why are they important in chemistry? |  |
Ans. Redox reactions, short for reduction-oxidation reactions, are chemical processes that involve the transfer of electrons between two species. In these reactions, one substance is oxidized (loses electrons) while another is reduced (gains electrons). They are crucial in various chemical processes, including combustion, respiration, and corrosion, as well as in batteries and electrochemical cells.
| 2. How can you identify oxidation and reduction in a redox reaction? |  |
Ans. In a redox reaction, oxidation can be identified by an increase in the oxidation state of an element, while reduction is indicated by a decrease in oxidation state. A helpful rule is to look for changes in the number of electrons associated with the atoms. For example, if iron (Fe) is oxidized from Fe²⁺ to Fe³⁺, it is losing an electron and is oxidized, whereas if copper (Cu²⁺) is reduced to Cu, it gains an electron.
| 3. What is the significance of oxidation states in balancing redox reactions? |  |
Ans. Oxidation states provide a systematic way to track the transfer of electrons in redox reactions. By assigning oxidation states to all elements in a reaction, chemists can determine which elements are oxidized and reduced, allowing for accurate balancing of the reaction. This balancing is essential for ensuring that the law of conservation of mass is upheld in chemical equations.
| 4. What are some common examples of redox reactions that are relevant in everyday life? |  |
Ans. Common examples of redox reactions include the rusting of iron, which involves the oxidation of iron in the presence of oxygen and moisture; photosynthesis, where carbon dioxide is reduced to glucose; and cellular respiration, where glucose is oxidized to produce energy. Additionally, the reactions in batteries, where chemical energy is converted to electrical energy, are also redox processes.
| 5. How are redox reactions applied in industrial processes? |  |
Ans. Redox reactions play a vital role in various industrial processes, including the extraction of metals from ores (e.g., reduction of metal oxides), wastewater treatment (where reduction reactions help in removing pollutants), and in the production of energy through fuel cells. These applications leverage the ability of redox reactions to convert materials efficiently and sustainably.
Related Searches
















