Class 10 Exam > Class 10 Notes > Science Class 10 > Infographics: Chemical Equations
Infographics: Chemical Equations | Science Class 10 PDF Download
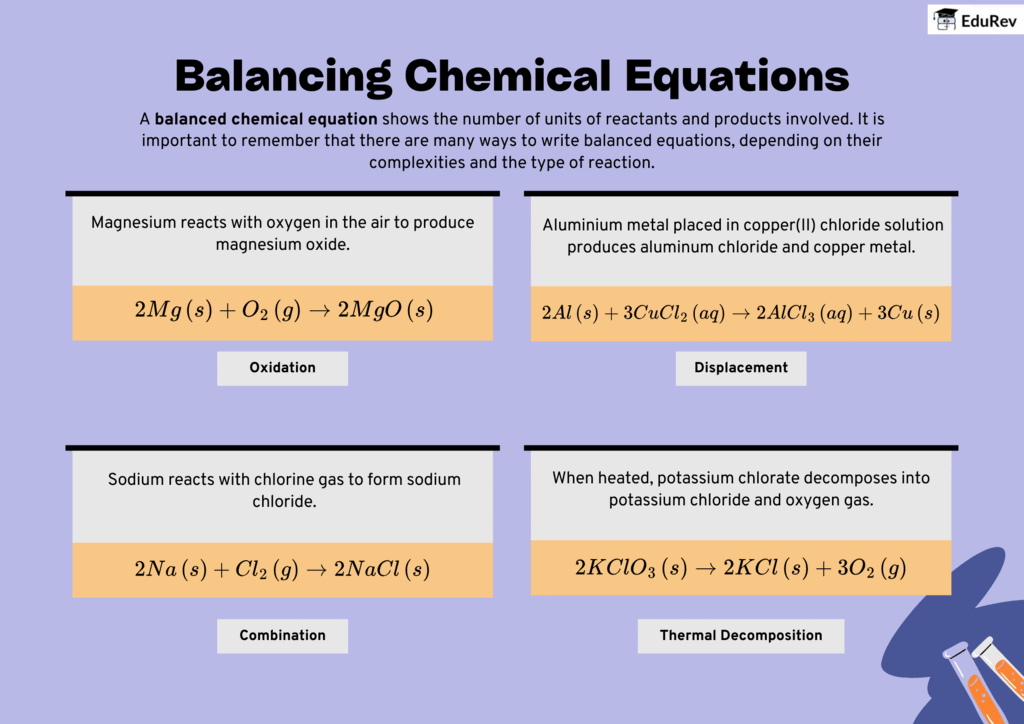
The document Infographics: Chemical Equations | Science Class 10 is a part of the Class 10 Course Science Class 10.
All you need of Class 10 at this link: Class 10
|
80 videos|569 docs|80 tests
|
FAQs on Infographics: Chemical Equations - Science Class 10
| 1. What are chemical equations and why are they important in chemistry? |  |
Ans. Chemical equations are symbolic representations of chemical reactions, showing the reactants (the substances that undergo the reaction) and the products (the substances formed). They are important because they provide a concise way to convey what happens during a chemical reaction, allowing chemists to understand the relationships between substances, predict the outcomes of reactions, and calculate quantities involved in reactions.
| 2. How do you balance a chemical equation? |  |
Ans. To balance a chemical equation, you must ensure that the number of atoms of each element is the same on both sides of the equation. This is done by adjusting the coefficients (the numbers in front of the compounds) without changing the chemical formulas. Start by balancing elements that appear in only one reactant and one product, then move on to more complex elements, and finally, balance hydrogen and oxygen last.
| 3. What is the difference between a balanced and unbalanced chemical equation? |  |
Ans. A balanced chemical equation has the same number of atoms of each element on both sides, which reflects the law of conservation of mass. An unbalanced equation does not have the same number of atoms for each element, meaning it does not accurately represent the reaction and can lead to incorrect predictions about the quantities of reactants and products.
| 4. Why do we use coefficients in chemical equations? |  |
Ans. Coefficients are used in chemical equations to indicate the relative amounts of reactants and products involved in a reaction. They help in balancing the equation and show the stoichiometry of the reaction, allowing chemists to calculate how much of each substance is needed or produced in a reaction.
| 5. Can you provide examples of types of chemical reactions represented in equations? |  |
Ans. Yes, common types of chemical reactions include synthesis (where two or more reactants combine to form one product), decomposition (where one compound breaks down into two or more products), single replacement (where one element replaces another in a compound), and double replacement (where the anions and cations of two different compounds exchange places). Each type can be represented by a chemical equation that illustrates the specific changes occurring during the reaction.
Related Searches






















