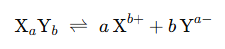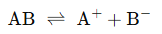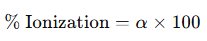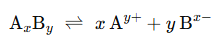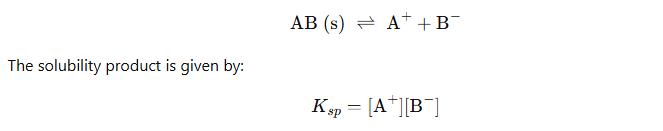Ionic Equilibrium, Ostwald’s Dilution Law & Related Concepts | Chemistry Class 11 - NEET PDF Download
What is Ionic Equilibrium?
In a polar solvent, an ionic substance dissociates into its constituent ions. These ions remain in equilibrium with the undissociated solute. The general representation is:
Equilibrium Characteristics:
In any equilibrium reaction, reactants and products coexist. The conversion of reactants to products is never 100%.Types of Reactions:
Equilibrium can involve the decomposition of covalent (non-polar) substances or the ionization of ionic compounds in polar solvents.
Classification Based on Electrical Conductivity
Non-Electrolytes
- Description: Consist of neutral molecules.
- Characteristics: Do not dissociate into ions; hence, they do not conduct electricity in aqueous or molten states.
- Example: Sugar solution.
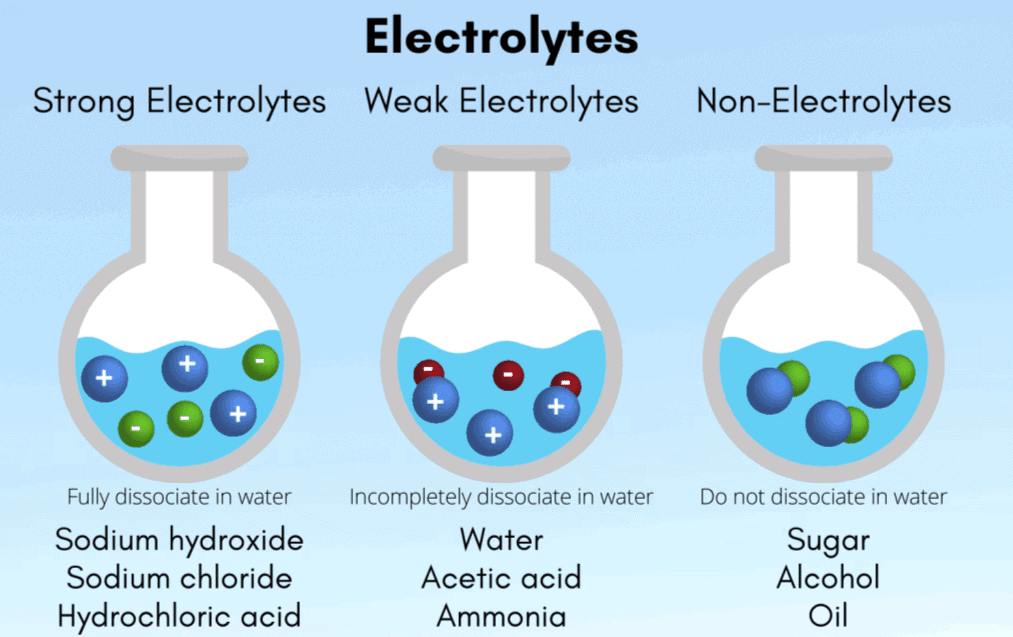
Electrolytes
- Description: Substances that dissociate into ions in solution.
- Characteristics: Conduct electricity in aqueous or molten states.
- Examples: Salt solution, acid solution, base solution.
Electrolytes are further classified into:
Strong Electrolytes:
Dissociate completely in solution (e.g., NaCl → Na⁺ + Cl⁻). The dissociation reaction is essentially one-way.Weak Electrolytes:
Dissociate partially; a reversible equilibrium is established between the ions and the undissociated molecules (e.g., acetic acid ionizing to give CH₃COO⁻ and H⁺).
Ostwald’s Dilution Law and Degree of Dissociation
Ostwald’s Dilution Law
This law applies the mass action principle to weak electrolytes. For a binary electrolyte AB that dissociates as follows:
Let:
- C = initial concentration,
- α = degree of dissociation (fraction of molecules dissociated).
For very weak electrolytes (), we approximate:
The equilibrium constant is given by: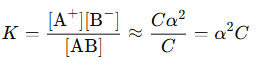
Thus, the degree of dissociation is: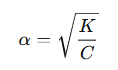
and the concentration of any ion becomes: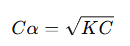
Key Point:
As the solution is diluted (i.e., as decreases), the degree of dissociation α increases. In other words, the degree of ionization is inversely proportional to the square root of the concentration.
Limitations of Ostwald’s Dilution Law
- Applicability:
The law is valid only for weak electrolytes. - Strong Electrolytes:
For strong electrolytes (which ionize completely), the law does not hold.
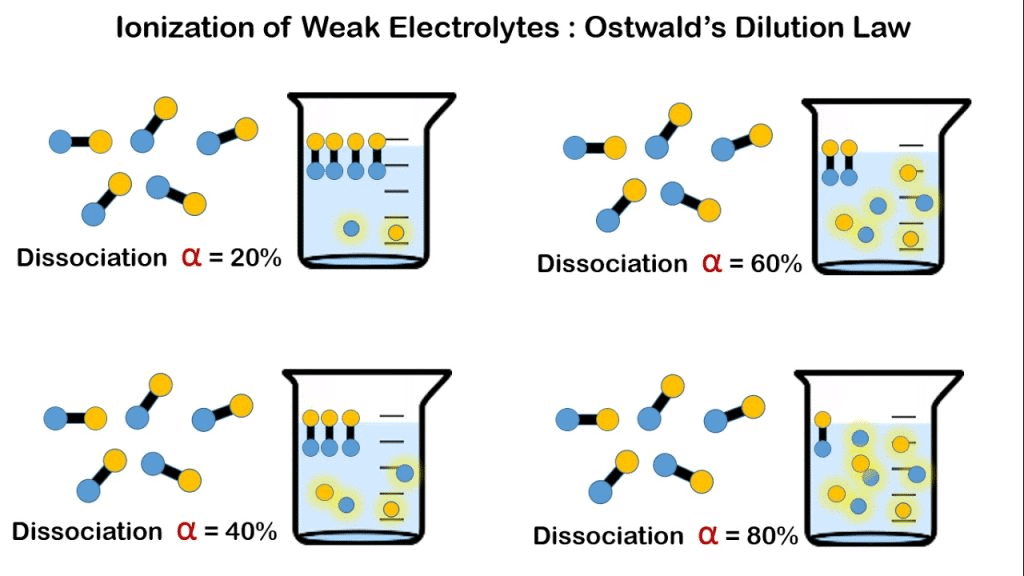
Degree of Dissociation (Ionization)
The degree of dissociation or ionization (α) indicates the fraction of the initial molecules that are converted into ions at equilibrium.
It can also be expressed as a percentage:
Factors Affecting Ionization
- Nature of the Electrolyte:
Whether the electrolyte is strong, weak, or sparingly soluble. - Nature of the Solvent:
Solvents with high dielectric constants (high polarity) favor ionization. - Dilution:
Greater dilution increases ionization. - Temperature:
Higher temperatures generally increase ionization. - Common Ion Presence:
The presence of a common ion decreases the ionization of a weak electrolyte.
Dissociation of Ionic Compounds in Polar Solvents
When ionic compounds dissolve in polar solvents (e.g., water), they ionize into cations and anions. However, these ions remain in equilibrium with the undissociated molecules:
Ionic Solids in Solutions
- Strong Electrolytes: Almost 100% ionization (e.g., HCl, NaOH).
- Weak Electrolytes: Approximately 10% ionization (e.g., acetic acid).
- Sparingly Soluble Salts: May ionize completely when they dissolve, but their overall solubility is low (e.g., AgCl, BaSO₄).
Ionization of Weak Electrolytes
- Infinite Dilution: Even weak electrolytes become fully ionized at infinite dilution.
- Concentrated Solutions: Weak electrolytes exist in a dynamic equilibrium with their undissociated molecules, affecting properties such as acid-base behavior and electrical conductance.
Common Ion Effect
The common ion effect describes the suppression of ionization of a weak electrolyte when an ion already present in the solution is added from another source. This is a direct consequence of Le Chatelier’s principle.
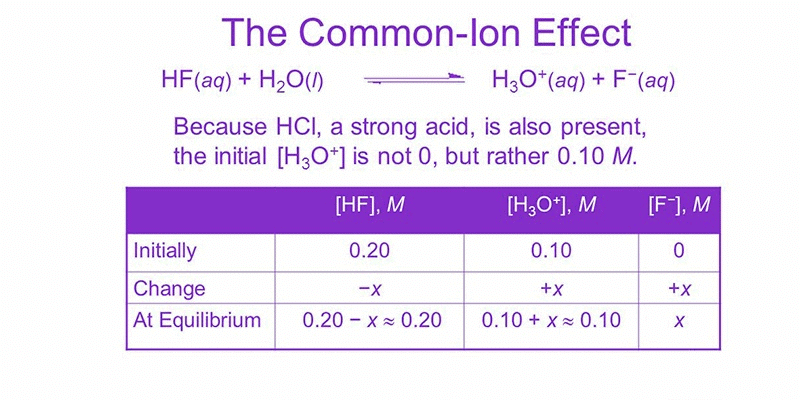
- In a solution with several species in equilibrium, increasing the concentration of one of the dissociated ions by adding a salt containing that ion shifts the equilibrium toward the undissociated form.
Examples of Common Ion Effect
- Ammonium hydroxide is a weak base. On addition of ammonium chloride (a salt) ammonium ion from it, will make the ammonium ions to combine with the hydroxide to form unionized ammonium hydroxide.
NH4Cl → NH4+ + Cl–
NH4OH ⇌ NH4+ + OH– - In the base hydrolysis of oil, the sodium salt of the fatty acid (soap) is in a dissolved state. When sodium chloride salt is added, the concentration of Na+ ions increases considerably.
CnH2n+1 + COONa ⇌ CnH2n+1 COO– + Na+
NaCl ⇌ Na+ + Cl–
Hence, the ionic product [CnH2n+1COO–] [Na+] exceeds the solubility product of soap and, therefore, soap precipitates out from the solution. This is called salting out of soap. - Manufacture of sodium bicarbonate (baking soda): In Solvay’s soda process. The CO2 gas is passed through ammonical brine to precipitate out NaHCO3.
NH4OH + CO2 → NH4HCO3
NH4HCO3 + NaCl → NH4HCO3 + NH4Cl
NaHCO3 is precipitated first because of its lower solubility product as compared to those of NH4Cl, NH3HCO3 and NaCl.
pH and Buffer Systems
When the conjugate ion of a buffer is added to the system, the common ion effect alters the equilibrium. For example, in a solution of acetic acid (a weak acid) and sodium acetate (a strong electrolyte):
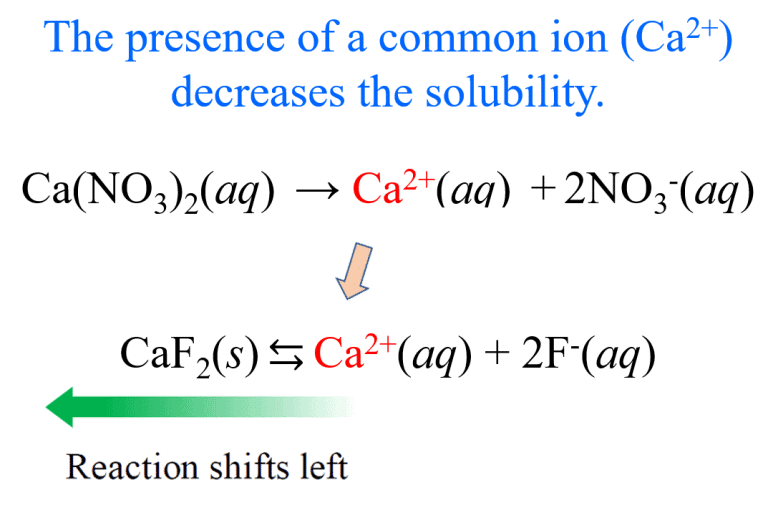
- Acetic Acid Equilibrium:
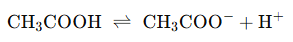
- Effect of Adding Sodium Acetate:
Sodium acetate dissociates completely, increasing the CH3COO− concentration. This shifts the equilibrium to the left, reducing the ionization of acetic acid and thereby increasing the pH (making the solution less acidic).
Solubility Equilibria and Solubility Product (Ksp)
Definition of Solubility Product (Ksp)
The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. It is denoted by the symbol Ksp.
- The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
- Solubility is defined as a property of a substance called solute to get dissolved in a solvent in order to form a solution. The solubility of ionic compounds (which disassociate to form cations and anions) in water varies to a great deal.
- Some compounds are highly soluble and may even absorb moisture from the atmosphere whereas others are highly insoluble.
Significance of Solubility Product
Solubility depends on a number of parameters amongst which lattice enthalpy of salt and solvation enthalpy of ions in the solution are of most importance.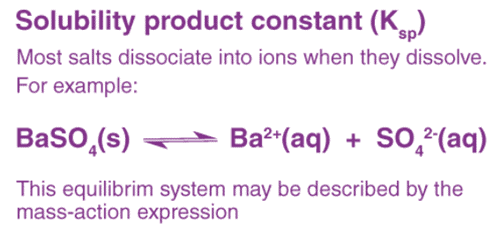
- When a salt is dissolved in a solvent the strong forces of attraction of solute (lattice enthalpy of its ions) must be overcome by the interactions between ions and the solvent.
- The solvation enthalpy of ions is always negative which means that energy is released during this process.
- The nature of the solvent determines the amount of energy released during solvation that is solvation enthalpy.
- Non-polar solvents have a small value of solvation enthalpy, meaning that this energy is not sufficient to overcome the lattice enthalpy.
- So the salts are not dissolved in non-polar solvents. Hence, for salt to be dissolved in a solvent, its solvation enthalpy should be greater than its lattice enthalpy.
- Solubility depends on temperature and it is different for every salt.
Salts are classified on the basis of their solubility in the following table:
Solubility Product Constant
Suppose barium sulphate along with its saturated aqueous solution is taken.
The following equation represents the equilibrium set up between the undissolved solids and ions:
The equilibrium constant in the above case is: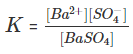
In case of pure solid substances the concentration remains constant, and so we can say: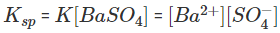
Here Ksp is known as the solubility product constant. This further tells us that solid barium sulphate when in equilibrium with its saturated solution, the product of concentrations of ions of both barium and sulphate is equal to the solubility product constant.
Factors affect the value of Ksp
Some important factors that have an impact on the solubility product constant are:
- The common-ion effect (the presence of a common ion lowers the value of Ksp).
- The diverse-ion effect (if the ions of the solutes are uncommon, the value of Ksp will be high).
- The presence of ion-pairs.
Conclusion
Understanding ionic equilibrium, Ostwald’s dilution law, and solubility product concepts is essential for mastering topics in physical chemistry. The interplay between ionization, dilution, common ion effect, and solubility equilibria not only explains many laboratory phenomena but also has significant practical applications—from water treatment to industrial synthesis processes.
|
114 videos|263 docs|74 tests
|
FAQs on Ionic Equilibrium, Ostwald’s Dilution Law & Related Concepts - Chemistry Class 11 - NEET
| 1. What is ionic equilibrium in solution? |  |
| 2. How does ionic equilibrium affect pH? |  |
| 3. What factors can disrupt ionic equilibrium in a solution? |  |
| 4. How can we calculate the degree of dissociation in an ionic equilibrium? |  |
| 5. What are some common examples of ionic equilibrium in everyday life? |  |