Isomerism in Alkanes, Alkenes & Alkynes | Chemistry Class 11 - NEET PDF Download
Isomerism
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulae but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers.
The word “isomer” is derived from the Greek words “isos” and “meros”, which mean “equal parts”. This term was coined by the Swedish chemist Jacob Berzelius in the year 1830.
Types
There are two primary types of isomerism, which can be further categorized into different subtypes. These primary types are Structural Isomerism and Stereoisomerism. The classification of different types of isomers is illustrated below.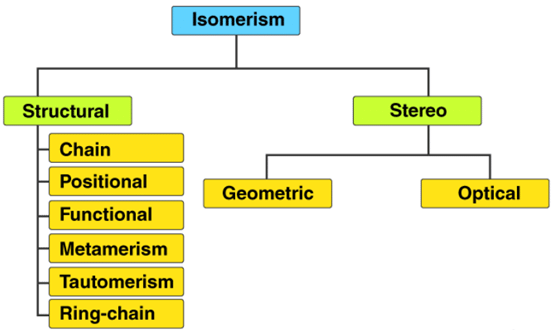
Structural Isomerism
Structural isomerism is commonly referred to as constitutional isomerism. The functional groups and the atoms in the molecules of these isomers are linked in different ways. Different structural isomers are assigned different IUPAC names since they may or may not contain the same functional group.
The different types of structural isomerism are discussed in this subsection:
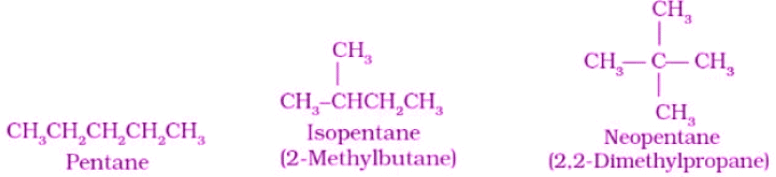
1. Chain Isomerism
- It is also known as skeletal isomerism.
- The components of these isomers display differently branched structures.
- Commonly, chain isomers differ in the branching of carbon.
- An example of chain isomerism can be observed in the compound C5H12, as illustrated below.
 2. Position Isomerism
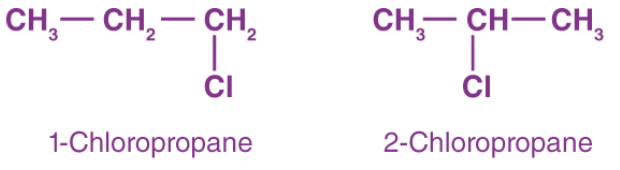
2. Position Isomerism
- The positions of the functional groups or substituent atoms are different in position isomers.
- Typically, this isomerism involves the attachment of the functional groups to different carbon atoms in the carbon chain.
- An example of this type of isomerism can be observed in the compounds having the formula C3H7Cl.
 3. Functional Isomerism
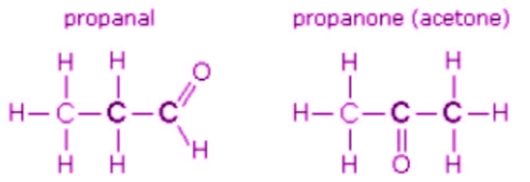
3. Functional Isomerism
- It is also known as functional group isomerism.
- As the name suggests, it refers to the compounds that have the same chemical formula but different functional groups attached to them.
- An example of functional isomerism can be observed in the compound C3H6O.
 4. Metamerism
4. Metamerism
- This type of isomerism arises due to the presence of different alkyl chains on each side of the functional group.
- It is a rare type of isomerism and is generally limited to molecules that contain a divalent atom (such as sulfur or oxygen), surrounded by alkyl groups.
- Example: C4H10O can be represented as ethoxyethane (C2H5OC2H5) and methoxy-propane (CH3OC3H7).
5. Tautomerism
- A tautomer of a compound refers to the isomer of the compound which only differs in the position of protons and electrons.
- Typically, the tautomers of a compound exist together in equilibrium and easily interchange.
- It occurs via an intramolecular proton transfer.
- An important example of this phenomenon is Keto-enol tautomerism.
6. Ring-Chain Isomerism
- In ring-chain isomerism, one of the isomers has an open-chain structure whereas the other has a ring structure.
- They generally contain a different number of pi bonds.
- A great example of this type of isomerism can be observed in C3H6. Propene and cyclopropane are the resulting isomers, as illustrated below.
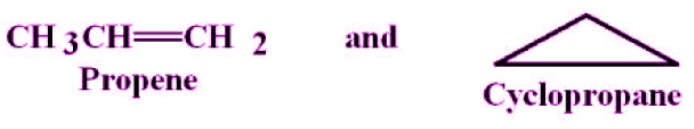 Stereoisomerism
Stereoisomerism
This type of isomerism arises in compounds having the same chemical formula but different orientations of the atoms belonging to the molecule in three-dimensional space. The compounds that exhibit stereoisomerism are often referred to as stereoisomers. This phenomenon can be further categorized into two subtypes. Both these subtypes are briefly described in this subsection.
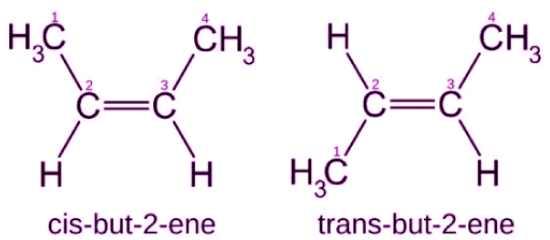
1. Geometric Isomerism
- It is popularly known as cis-trans isomerism.
- These isomers have different spatial arrangements of atoms in three-dimensional space.
- An illustration describing the geometric isomerism observed in the acyclic But-2-ene molecule is provided below.
2. Optical Isomerism
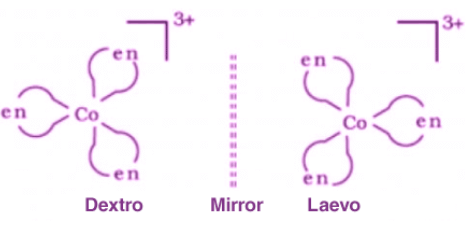
- Compounds that exhibit optical isomerism feature similar bonds but different spatial arrangements of atoms forming non-superimposable mirror images.
- These optical isomers are also known as enantiomers.
- Enantiomers differ from each other in their optical activities.
- Dextro enantiomers rotate the plane of polarized light to the right whereas laevo enantiomers rotate it to the left, as illustrated below.
|
114 videos|263 docs|74 tests
|
FAQs on Isomerism in Alkanes, Alkenes & Alkynes - Chemistry Class 11 - NEET
| 1. What is isomerism in alkanes, alkenes, and alkynes? |  |
| 2. How many types of isomerism exist in alkanes, alkenes, and alkynes? |  |
| 3. Can you provide an example of structural isomerism in alkanes? |  |
| 4. What is cis-trans isomerism in alkenes? |  |
| 5. How does isomerism affect the properties of alkanes, alkenes, and alkynes? |  |
















