Lakhmir Singh & Manjit Kaur: Carbon And Its Compounds, Solutions- 4 | Science Class 10 PDF Download
(Page No - 263)
Question 20:
Complete and balance the following equations :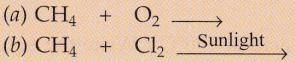
Solution :
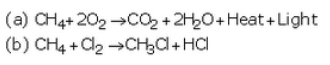
Question 21:
Fill in the following blanks with suitable words :
(a) The process of burning of a hydrocarbon in the presence of air to give CO2, H2O, heat and light is known as………
(b) The sodium salt of a long chain fatty acid is called………………..
(c) is better than soap for washing clothes when the water is hard.
(d) The organic acid present in vinegar is………………..
Solution :
(a) Combustion
(b) Soap
(c) Detergent
(d) Ethanoic acid
Question 22:
Which of the following hydrocarbons will give substitution reactions and why ?
CH4, C3H6, C3H8, C4H6, C5H12, C5H10
Solution :
CH4, C3H8 and C5H12; all these are saturated hydrocarbons (Alkanes) and hence will give substitution reactions.
Solution 23
C2H4 and C3H4 will give addition reactions because these are unsaturated hydrocarbons (Alkene and Alkyne) and unsaturated hydrocarbons give addition reactions.
Question 24:
(a) Write the chemical equation of the reaction which takes place during the burning of ethanol in air.
(b) Why is ethanol used as a fuel ?
(c) State two uses of ethanol (other than as a fuel).
Solution :
(b) Since, ethanol burns with a clear flame giving a lot of heat, therefore, it is used as a fuel.
(c) Uses of ethanol:
(i) It is used in the manufacture of paints, varnishes, lacquers, medicines, perfumes, dyes, soaps and synthetic rubber.
(ii) It is used as a solvent. Many organic compounds which are insoluble in water are soluble in ethyl alcohol.
Question 25:
(a) What happens when propanoic acid is warmed with methanol in the presence of a few drops of
concentrated sulphuric acid ? Write equation of the reaction involved.
(b) What change will you observe if you test soap solution with a litmus paper (red and blue) ? Give reason for your observation.
(c) What is meant by denatured alcohol ? What is the need to denature alcohol ?
Solution :
(a) Propanoic acid will react with the alcohol in the presence of concentrated sulphuric acid to form esters.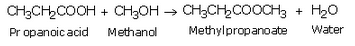
(b) Red litmus paper turns blue in soap solution and no change occurs on blue litmus paper because soap is basic in nature.
(c) Denatured alcohol is ethyl alcohol which has been made unfit for drinking purposes by adding small amounts of poisonous substances like methanol, pyridine, copper sulphate etc. This is done to prevent the misuse of industrial alcohol for drinking purposes or black marketing (as it is supplied duty free for industrial purposes by the government).
Question 26:
(a) How would you test for an alcohol ?
(b) Give the harmful effects of drinking alcohol.
(c)Explain why, methanol is much more dangerous to drink than ethanol.
Solution :
(a) Sodium metal test: Add a small piece of sodium metal to the organic liquid (to be tested), taken in a dry test tube. If bubbles (or effervescence) of hydrogen gas are produced, it indicates that the given organic liquid is an alcohol.
(b) Harmful effects of drinking alcohol:
(i) Alcohol slows down the activity of the nervous system and brain due to which the judgement of a person is impaired and his reaction becomes slow.
(ii) Heavy drinking of alcohol on a particular occasion leads to staggered movement, slurred speech and vomiting.
(c) Unlike ethanol, drinking methanol, even in a small quantity can be fatal leading to permanent blindness and even death. Methanol damages the optic nerve causing permanent blindness in a person. This happens because methanol is oxidised to methanal in the liver of a person. This methanal reacts rapidly with the components of the cell causing coagulation of their protoplasm. Due to this, the cells stop functioning normally.
Question 27:
How would you convert:
(a) ethanol into ethene ?
(b) propanol into propanoic acid ?
Name the process in each case and write the equations of the reactions involved.
Solution :
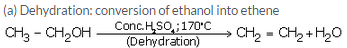
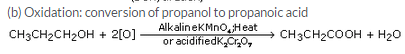
Question 28:
Give reasons for the following observations :
(a) Air holes of a gas burner have to be adjusted when the vessels being heated get blackened by the flame.
(b) Use of synthetic detergents causes pollution of water.
Solution :
(a) Air holes of a gas burner have to be adjusted b ecause blackening of vessels show that the air holes of the gas stove are getting blocked and hence the fuel is not burning completely (due to insufficient supply of oxygen).
(b) Some of the detergents (synthetic) are not bio-degradable, that is they cannot be decomposed by micro organisms like bacteria and hence cause water pollution.
Question 29:
(a) What would be observed on adding a 5% alkaline potassium permanganate solution drop by drop to
some warm ethanol in a test-tube ? Write the name of the compound formed during the chemical reaction. Also write chemical equation of the reaction which takes place.
(b) How would you distinguish experimentally between an alcohol and a carboxylic acid on the basis of a chemical property ?
Solution :
(a) On adding 5% alkaline potassium permanganate solution drop by drop to some warm ethanol, we would observe that the purple color of potassium permanganate starts disappearing; the product formed by this process; ethanoic acid can turn blue litmus red.
(b) A carboxylic acid reacts with sodium hydrogencarbonate to give brisk effervescence of carbon dioxide gas but an alcohol does not react with sodium hydrogencarbonate.
Question 30:
Name the functional group of organic compounds that can be hydrogenated. With the help of a suitable example, explain the process of hydrogenation, mentioning the conditions of the reaction and any one change in physical property with the formation of the product. Name any one natural source of organic compounds that are hydrogenated.
Solution :
Alkenes can be hydrogenated.
The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon is called hydrogenation.
Example: Ethene reacts with hydrogen in the presence of finely divided nickel as catalyst to form ethane.
Liquid vegetable oils are hydrogenated into vegetable ghee (solid fat).

Question 31:
(a) Name the gas evolved when ethanol reacts with sodium.
(b) What type of compound is formed when a carboxylic acid reacts with an alcohol in the presence of cone. H2SO4 ?
(c) What will you observe when dilute ethanoic acid and dilute hydrochloric acid are put on universal indicator paper, one by one ? What does it show ?
Solution :
(a) Hydrogen gas is evolved when ethanol reacts with sodium.
(b) Esters are formed when a carboxylic acid reacts with an alcohol in the presence of conc. H2SO4.
(c) Dilute ethanoic acid turns universal indicator paper to orange, showing that its pH is about 4 which tell us that ethanoic acid is a weak acid. On the other hand, dilute hydrochloric acid turns universal indicator paper to red, showing that its pH is about 1. This shows us that hydrochloric acid is a strong acid.
Question 32:
(a) What type of compound is CH3COOH ?
(b) What substance should be oxidised to prepare CH3COOH ?
(c) What is the physical state of CH3COOH ?
(d) State one advantage of soaps over detergents.
Solution :
(a) CH3COOH is a c arboxylic acid.
(b) Ethanol, CH3CH2OH should be oxidised to prepare CH3COOH.
(c) Liquid state
(d) Soaps are biodegradable whereas detergents are non-biodegradable.
(Page No - 264)
Question 33:
(a) What happens when ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid ? Write equation of the reaction involved.
(b) What happens when ethanol is heated with concentrated sulphuric acid at 170°C ? Write the equation of the reaction which takes place.
Solution :
(a) When ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid, a sweet smelling ester called ethyl ethanoate is formed.
(b) When ethanol is heated with concentrated sulphuric acid at 1700-C, it gets dehydrated to form ethene.
Question 34:
(a) What happens when ethanol is oxidised with alkaline potassium permanganate (or acidified potassium dichromate) ? Write the equation of the reaction involved.
(b) Choose those compounds from the following which can turn blue litmus solution red :
HCHO, CH3COOH, CH3OH, C2H5OH, HCOOH, CH3CHO Give reasons for your choice.
Solution :
(a) When ethanol is oxidised with alkaline potassium permanganate (or acidified potassium dichromate), it gets oxidised to form ethanoic acid.
(b) CH3COOH and HCOOH can turn blue litmus solution red. These are organic acids.
Question 35:
(a) Explain the process of preparation of soap in laboratory.
(b) Why is common salt (sodium chloride) added during the preparation of soap ?
(c) Why is soap not suitable for washing clothes when the water is hard ?
Solution :
(a) Soap can be prepared in the laboratory as follows:
1. Take about 20 ml of castor oil (cottonseed oil, linseed oil or soya bean oil) in a beaker.
2. Add 30 ml of 20% sodium hydroxide solution to it.
3. Heat the mixture with constant stirring till a paste of soap is formed.
4. Then add 5 to 10 grams of common salt (sodium chloride).
5. Stir the mixture well and allow it to cool. On cooling the solution, solid soap separates out.
6. When the soap sets, it can be cut into pieces called ‘soap bars’.
(b) Common salt is added to the mixture to make the soap come out of solution. Though most of the soap separates out on its own but some of it remains in solution. Common salt is added to precipitate out all the soap from the aqueous solution.
(c) When soap is used for washing clothes with hard water, a large amount of soap in water is reacting with the calcium and magnesium ions of hard water to form an insoluble precipitate called scum, before it can be used for the real purpose of washing.
Question 36:
(a) What happens when methane (natural gas) burns in air ? Write the chemical equation of the reaction involved.
What happens when ethanoic acid reacts with sodium carbonate ? Write chemical equation of the reaction involved.
Give a test that can be used to differentiate chemically between butter and cooking oil.
Solution :
(a) Carbon dioxide and water vapour are formed when methane burns in air.
(b) Ethanoic acid reacts with sodium carbonate to form sodium ethanoate and carbon dioxide.
(c) Add bromine water to a little of cooking oil and butter taken in separate test tubes:
(i) Cooking oil decolourises bromine water (showing that it is an unsaturated compound). (ii) gutter does not decolourise bromine water (showing that it is a saturated compound).
Question 37:
(a) Describe, giving equation, a chemical reaction which is characteristic of saturated hydrocarbons (or alkanes).
What is an oxidising agent ? Name two oxidising agents which can oxidise ethanol to ethanoic acid.
Describe one reaction of a carboxylic acid.
Solution :
(a) Substitution reaction of methane with chlorine: Methane reacts with chlorine in the presence of sunlight to form chloromethane and hydrogen chloride.
(b) An oxidising agent is one which oxidises other substances by providing oxygen or removing hydrogen. Alkaline potassium permanganate and acidified potassium dichromate can be used as oxidising agents.
(c) Reaction with alcohols: Ethanoic acid reacts with alcohols in the presence of a little of conc. sulphuric acid to form esters.
Question 38:
(a) Write names and formulae of hydrocarbons containing a single and a double bond (one example for each). Give one characteristic chemical property of each.
(b) What is a detergent ? Name one detergent.
(c) Why have detergents replaced soap as a washing agent ?
Solution :
(a) (i) Single bond: Methane, CH4. They are quite unreactive hence they undergo substitution reaction with chlorine in presence of sunlight.
(ii) Double bond: Ethene, CH2=CH2. They undergo addition reaction in the presence of a catalyst like nickel or palladium.
(b) A detergent is the sodium salt of long chain benzene sulphonic acid which has cleansing properties in water. Ex: Sodium n-dodecyl benzene sulphonate.
(c) Detergents are better cleansing agents than soaps because they do not form insoluble calcium and magnesium salts with hard water, and hence can be used for washing even with hard water.
Question 39:
(a) How does ethanoic acid react with sodium hydrogencarbonate ? Give equation of the reaction which takes place.
(b) Why are carbon and its compounds used as fuels for most applications ?
Which of the two is better for washing clothes when the water is hard : soap or detergent ? Give reason for your answer.
Solution :
(a) Ethanoic acid reacts with sodium hydrogencarbonate to evolve brisk effervescence of carbon dioxide gas.
CH3COOH+ NaHCO3 → CH3COONa+ CO2 + H2O
(b) Carbon and its compounds used as fuels because they burn in air releasing a lot of heat energy.
(c) Detergent is better for washing clothes with hard water. They are better cleansing agents than soaps because they do not form insoluble calcium and magnesium salts with hard water, and hence can be used for washing even Wth hard water.
Question 40:
(a) What is meant by a substitution reaction ? Give an example (with equation) of the substitution reaction of, an alkane.
(b) How is soap made ? Write a word equation involved in soap making.
Solution :
(a) The reaction in which one (or more) hydrogen atoms of a hydrocarbon are replaced by some other atoms (like chlorine), is called a substitution reaction.
Example: Substitution reaction of methane with chlorine:- Methane reacts Wth chlorine in the presence of sunlight to form chloromethane and hydrogen chloride.
(b) Soap is made by heating animal fat or vegetable oil with concentrated sodium hydroxide solution.
Question 41:
(a) How is ethanoic acid obtained from ethanol ? Write down the chemical equation of the reaction involved.
(b) How would you distinguish between ethanol and ethanoic acid by chemical test ?
(c) Explain the formation of scum when hard water is treated with soap.
Solution :
(a) Ethanoic acid is obtained from ethanol by the means of oxidation reaction. When ethanol is heated with alkaline potassium permanganate solution (or acidified potassium dichromate solution), it gets oxidised to ethanoic acid. It is called an oxidation reaction because oxygen is added to it during this conversion.
(b) Litmus test: Some blue litmus solution is added to the organic compound (to be tested). If the blue litmus solution turns red, it shows that the organic compound is acidic in nature and hence it is a carboxylic acid (ethanoic acid). Ethanol has no effect on any litmus solution.
(c) When soap is used for washing clothes with hard water, a large amount of soap in water reacts with the calcium and magnesium ions of hard water to form an insoluble precipitate called scum. This makes the cleaning of clothes difficult.
Question 42:
(a) What happens when methane reacts with chlorine ? Give equation of the reaction which takes place.
(b) What is hydrogenation ? What is its industrial application ?
(c) Give any two differences between soaps and detergents.
Solution :
(a) Methane reacts with chlorine in the presence of sunlight to form chloromethane and hydrogen chloride. This reaction is called substitution reaction.
(b) The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon is called hydrogenation. Application: Vegetable oils are hydrogenated to form vegetable ghee (or vanasapati ghee).
(c)
| Soaps | Detergents |
| (i) Soaps are biodegradable. (ii) Soaps have relatively weak cleansing action. | (i) Detergents are not biodegradable (ii) Detergents have a strong cleansing action |
Question 43:
(a) What happens when ethanoic acid reacts with sodium hydroxide ? Write equation of the reaction involved.
(b) What happens when vegetable oils are hydrogenated ? Name the catalyst used.
(c) What is the advantage of detergents over soaps for washing clothes ? Also state one disadvantage.
Solution :
(a) Ethanoic acid reacts with sodium hydroxide to form a salt called sodium ethanoate and water.
CH3COOH+NaOH → CH3COONa+H2O
(b) On hydrogenation, the liquid vegetable oils change into solid fat (vanasapati ghee). Nickel or palladium can be used as the catalyst.
(c) Advantage: Detergents can be used even with hard water and have stronger cleaning action.
Disadvantage: Detergents are not biodegradable and hence cause water pollution.
Question 44:
(a) An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogencarbonate. Give the name and formula of X.
(b) A mixture of ethyne (acetylene) and oxygen is burnt for welding. Can you tell why a mixture of ethyne and air is not used ?
(c) Name a chemical reaction which is characteristic of unsaturated hydrocarbons (like alkenes and alkynes).
Solution :
(a) Ethanoic acid, CH3COOH gives brisk effervescence with sodium hydrogencarbonate.
(b) A mixture of ethyne and air is not used for welding because burning of ethyne in air produces a sooty flame (due to incomplete combustion) which is not hot enough to melt metals for welding.
(c) Addition reactions are a characteristic of unsaturated hydrocarbons.
Question 45:
(a) What is meant by an addition reaction ? Give an example (with equation) of an addition reaction of an alkene.
(b) What is added to groundnut oil when it is to be converted to vanaspati ghee ?
(c) Which of the two is better for our health : butter or vegetable oil ? Why ?
Solution :
(a) The reaction in which an unsaturated hydrocarbon combines with another substance to give a single product is called an addition reaction.
Example: Ethene reacts with hydrogen when heated in the presence of nickel catalyst to form ethane:
(b) Hydrogen is added to groundnut oil when it is to be converted to vanaspati ghee.
(c) Vegetable oil is better because it has unsaturated fatty acids v.hich are good for our health.
(Page No - 265)
Question 46:
(a) When ethanoic acid reacts with sodium hydrogencarbonate, then a salt X is formed and a gas Y is evolved.
Name the salt X and gas Y. Describe an activity with the help of a labelled diagram of the apparatus used to prove that the evolved gas is the one which you have named. Also write the chemical equation of the reaction involved.
(b) Give any two uses of ethanoic acid.
Solution :
(a)Salt X is sodium ethanoate, CH3COONa; Gas Y is carbon dioxide, CO2
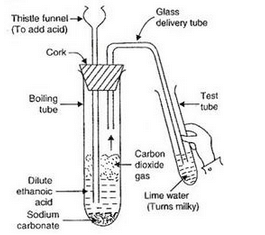
Activity: Take a boiling tube and put about 0.5 g of sodium carbonate in it. Add 2 ml of dilute ethanoic acid to the boiling tube (through a thistle funnel). We will observe that brisk effervescence of carbon dioxide gas is produced. Let us pass this gas through lime water taken in a test tube. We will find that lime water turns milky. Only carbon dioxide gas can turn lime water milky. So, this experiment proves that when ethanoic acid reacts with sodium carbonate, then carbon dioxide gas is evolved.
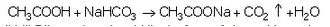
(b)(i) Dilute ethanoic acid (in the form of vinegar) is used as a food preservative in the preparation of pickles and sauces.
(ii) It is used in the manufacture of acetone and esters used in perfumes.
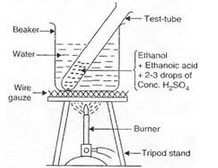
(a) Activity:
(i) Take 1 ml of pure ethanol (absolute alcohol) in a test-tube and add 1 ml of glacial ethanoic acid to it. Then add 2 or 3 drops of concentrated sulphuric acid to the mixture.
(ii) Warm the test-tube containing above reaction mixture in hot water bath (a beaker containing hot water) for about 5 minutes.
(iii) Pour the contents of the test-tube in about 50 ml of water taken in another beaker and smell it.
(iv) A sweet smell is obtained indicating the formation of an ester.
Reaction:
(c) Uses of esters:
(i) Esters are used in making artificial flavours and essences. These are used in cold drinks, ice-creams, sweets and perfumes.
(ii) Esters are used as solvents for oils, fats, gums, resins, cellulose, paints, varnishes, etc.
(iii) Pour the contents of the test-tube in about 50 ml of water taken in another beaker and smell it.
(iv) A sweet smell is obtained indicating the formation of an ester.
Reaction:
Question 48:
(a) Name the reaction which is usually used in the conversion of vegetable oils to fats. Explain the reaction
involved in detail. Write a chemical equation to illustrate your answer.
(b) What is saponification ? Write the chemical equation of the reaction involved in this process. Name all the substances which take part in this process and also those which are formed.
(c) Why does micelle formation take place when soap is added to water ? Will a micelle be formed in other solvents like ethanol also ?
Solution :
(a) Catalytic hydrogenation is usually used in conversion of vegetable oils to fats. Hydrogenation of oils: Vegetable oils are unsaturated fats having double bonds between some of their carbon atoms and can undergo addition reactions. When a vegetable oil (like groundnut oil) is heated with hydrogen in the presence of finely divided nickel as catalyst, then a saturated fat called vegetable ghee (or vanaspati ghee) is formed. This reaction is called hydrogenation of oils and it can be represented as follow.

(b) The process of making soap by the hydrolysis of FatcrCl + Scdiumhydroxlde Scep + Glycerd fats and oils with alkalis called saponification.
(c) Soap are sodium or potassium salts of long-chain carboxylic acids. When soap is added to the water, the hydrophilic end (acid end) will align along the surface of water and the hydrophobic tail (carbon chain) remains out of water. When a soap is dissolved in water, it forms a colloidal suspension in water in which the soap molecules cluster together to form spherical aggregates called micelles. In a soap micelle, soap molecules are arranged radially Wth hydrocarbon ends directed towards the centre and ionic ends directed ounvards.
No, micelle will not be formed in other solvents such as ethanol because hydrocarbon chains of soap molecules are soluble in organic solvents like ethanol.
Question 49:
(a) What is a soap ? Name one soap.
(b) Describe the structure of a soap molecule with the help of a diagram.
(c) Explain the cleansing action of soap. Draw diagrams to illustrate your answer.
Solution :
(a) A soap is the sodium salt (or potassium salt) of a long chain carboxylic acid (fatty acid) which has cleansing properties in water.
Example: Sodium stearate, C17H35COO–Na+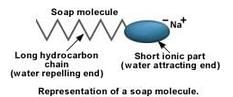
(b) A soap molecule has two parts: the long chain organic part and the ionic part containing the -COO–Na+group. It has to be remembered that this is not an ion, the atoms are all covalently bonded, the electrical charges show how the charges get polarized in the group. A soap molecule has a tadpole like structure shown below:
(c) Cleaning action of soap has been explained with the help of the image below:
Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is it dissolves in water, while the other end is hydrophobic, that is it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water.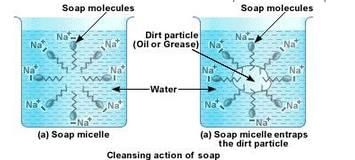
Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion inside the water. This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. When a dirty cloth is put in water containing dissolved soap, then soap in the form of a micelle is able to clean. The hydrocarbon ends of the soap attach to the oily dirt particles and entrap them at the centre of the micelle. the ionic ends in the micelles remain attached to water. When the dirty cloth is agitated in soap solution, the oily dirt particles entrapped by soap micelles get dispersed in water and the cloth gets cleaned.
(Page No - 266)
Question 66:
A neutral organic compound X of molecular formula C2H6O on oxidation with acidified potassium dichromate gives an acidic compound Y. Compound X reacts with Y on warming in the presence of cone. H2S04 to give a sweet smelling substance Z. What are X, Y and Z ?
Solution :
X is ethanol
Y is ethanoic acid
Z is ethyl ethanoate
Ethanol reacts with ethanoic acid to form ethyl ethanoate ester.
Question 67:
Consider the following organic compounds :
HCHO, C2H5OH, C2H6, CH3COOH, C2H5C1
Choose two compounds which can react in the presence of cone. H2S04 to form an ester. Give the name and formula of the ester formed.
Solution :
C2H5OH and CH3COOH react in the presence of conc. H2SO4 to form an ester. Ethyl ethanoate, CH3COOC2H5 is formed in the reaction.
Question 68:
A neutral organic compound is warmed with some ethanoic acid and a little of cone. H2S04. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound ? The structural formula of an ester is :
Solution :
Alcohol group, -OH. Acids react with alcohols to form sweet smelling esters.
Question 69:
The structural formula of an ester is :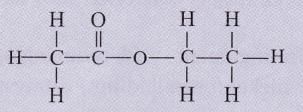
Write the formula of the acid and the alcohol from which it is formed.
Solution :
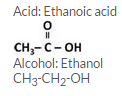
Question 70:
Consider the following organic compounds :
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO Out of these compounds :
(a) Which compound is most likely to be sweet-smelling ?
(b) Which compound on treatment with cone. H2S04 at 170°C forms an alkene ?
(c) Which compound on repeated chlorination forms chloroform ?
(d) Which compound is added to alcohol to denature it ?
(e) Which compound is a constituent of vinegar ?
(f) Which compound is used to sterilise wounds and syringes ?
Solution :
(a) C4H9COOC2H5; Ester
(b) C2H5OH; Alcohol forms ethene, C2H4
(c) CH4; Methane
(d) CH3OH; Methanol
(e) CH3COOH; Acetic acid
(f) C2H5OH; Ethanol
Question 71:
An organic acid X is a liquid, Which often freezes during winter time in cold countries, having the molecular formula C2H402. On warming it with methanol in the presence of a few drops of concentrated sulphuric acid, a compound Y with a sweet smell is formed.
(a) Identify X and Y. Also write their formulae showing the functional group present in them.
(b) Write a chemical equation for the reaction involved.
Solution :
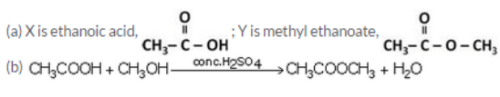
Question 72:
An organic compound A having the molecular formula C3H8O is a liquid at room temperature. The organic liquid A reacts with sodium metal to evolve a gas which burns causing a little explosion. When the organic liquid A is heated with concentrated sulphuric acid at 170°C, it forms a compound B which decolourises bromine water. The compound B adds on one molecule of hydrogen in the presence of Ni as catalyst to form compound C which gives substitution reactions with chlorine.
(a) What is compound A ?
(b) What is compound B ?
(c) What type of reaction occurs when A is converted into B ?
(d) What is compound C ?
(e) What type of reaction takes place when B is converted into C ?
Solution :
(a) A is propanol, CH3-CH2-CH2OH
(b) B is propene, CH3CH=CH2
(c) Dehydration reaction
(d) C is propane, CH3CH2-CH3
(e) Addition reaction
(Page No - 267)
Question 73:
An organic compound A (molecular formula C2H402) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of a little of concentrated sulphuric acid forms a sweet-smelling compound D (molecular formula C3H602). Compound D on treatment with NaOH solution gives back B and C. Identify A, B, C and D.
Solution :
A is ethanoic acid, CH3COOH
B is sodium ethanoate, CH3COONa
C is methanol, CH3OH
D is methyl ethanoate, CH3COOCH3
Question 74:
Which of the following hydrocarbons can decolourise bromine water and which cannot ? Why ?
C6H12, C6H14, C6H10
Solution :
C6H12 and C6H10 c an decolourise bromine water since these are unsaturated hydrocabons.
C6H14 cannot decolourise bromine water since it is a saturated hydrocarbon.
Question 75:
A four carbon atoms containing neutral organic compound X reacts with sodium metal to evolve a gas which burns with a ‘pop’ sound. Another four carbon atoms containing carbon compound reacts with sodium hydrogencarbonate to evolve a gas which turns lime water milky. When compounds X and Y are heated together in the presence of a little of concentrated sulphuric acid, then a new compound Z is formed.
(a) What is compound X ? Also write its formula.
(b) What is compound Y ? Also write its formula,.
(c) What is compound Z ? Also write its formula.
(d) What type of smell is given by compound Z ?
(e) What is the general name of compounds like Z ?
(f) What is the general name of the reaction which takes place between X and Y to form Z ?
Solution :
(a) X is butanol, C4H9OH
(b) Y is butanoic acid, C3H7COOH
(c) Z is butyl butanoate, C3H7COOC4H9
(d) Sweet smell is given by the compound Z.
(e) Esters
(f) Esterification reaction.
|
80 videos|569 docs|80 tests
|
FAQs on Lakhmir Singh & Manjit Kaur: Carbon And Its Compounds, Solutions- 4 - Science Class 10
| 1. What is the importance of carbon compounds in our daily life? |  |
| 2. What are the different types of carbon compounds? |  |
| 3. How does carbon form multiple bonds with other atoms? |  |
| 4. What are the greenhouse gases and their role in climate change? |  |
| 5. How do carbon compounds play a role in the carbon cycle? |  |
















