Lakhmir Singh & Manjit Kaur: Metals and Non-metals, Solutions- 2 | Science Class 10 PDF Download
(Page No - 134)
Question 46:
A copper plate was dipped in AgNO3 After certain time, silver from the solution was deposited on the copper plate. State the reason why it happened. Give the chemical equation of the reaction involved.
Solution :
Silver gets deposited on the copper plate because copper is more reactive than silver and hence displaces silver from silver nitrate solution.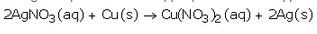
Question 47:
State five uses of metals and five of non-metals.
Solution :
Uses of metals:
(i) Lead metal is used in making car batteries.
(ii) Zinc is used for galvanizing iron to protect it from rusting.
(iii) Iron, copper and aluminium are used to make utensils.
(iv) Copper and aluminium metals are used to make electrical wires.
(v) Aluminium is used to make aluminium foil for packaging materials.
Uses of non-metals:
(i) Hydrogen is used in the hydrogenation of vegetable oils.
(ii) Carbon is used to make electrodes of electrolytic cells and dry cells.
(iii) Nitrogen is used in the manufacture of ammonia, nitric acid and fertilizers.
(iv) Sulphur is used for producing sulphuric acid.
(v) Liquid hydrogen is used as rocket fuel.
Question 48:
State one use each of the following metals :
Copper, Aluminium, Iron, Silver, Gold, Mercury
Solution :
(i) Copper – Copper is used to make wires to carry electric current.
(ii) Aluminium – Aluminium foils are used in packaging of food materials.
(iii) Iron – Iron is used to make utensils.
(iv) Silver – Silver is used to make jewellery.
(v) Gold – Gold is used to make jewellery.
(vi) Mercury – Mercury is used in thermometers.
Question 49:
(a) State one use each of the following non-metals :
Hydrogen, Carbon (as Graphite), Nitrogen, Sulphur
(b) Name the metal which is used in making thermometers.
Solution :
(a) (i) Hydrogen – Hydrogen is used in the hydrogenation of vegetable oils.
(ii) Carbon is used to make electrodes of electrolytic cells and dry cells.
(iii) Nitrogen is used in the manufacture of ammonia, nitric acid and fertilizers.
(iv) Sulphur is used in making sulphuric acid.
(b) Mercury
Question 50:
(a) Why does aluminium not react with water under ordinary conditions ?
(b) Name two metals which can displace hydrogen from dilute acids.
(c) Name two metals which cannot displace hydrogen from dilute acids.
Solution :
(a) Aluminium metal does not react with water under ordinary conditions because of the presence of a thin layer of aluminium oxide on its surface.
(b) Sodium and magnesium.
(c) Copper and silver.
Question 51:
(a) Why is sodium kept immersed in kerosene oil ?
(b) Why is white phosphorus kept immersed under water ?
(c) Can we keep sodium immersed under water ? Why ?
Solution :
(a) Sodium is a very reactive metal so it reacts vigorously with the oxygen of air and catches fire. It is kept immersed in kerosene oil to protect it from the action of oxygen, moisture and carbon dioxide of air and to prevent accidental fires.
(b) White phosphorus is kept immersed in water because it reacts spontaneously with oxygen of air to form phosphorus pentoxide but does not react with water.
(c) No, because sodium reacts vigorously with water to form sodium hydroxide and hydrogen.
Question 52:
(a) Describe the reaction of potassium with water. Write the equation of the reaction involved.
(b) Write an equation of the reaction of iron with steam. Indicate the physical states of all the reactants and products.
(c) Which gas is produced when dilute hydrochloric acid is added to a reactive metal ?
Solution :
(a) Potassium reacts violently with cold water to form potassium hydroxide and hydrogen gas.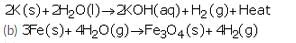
(c) Hydrogen.
Question 53:
(a) Give one example, with equation, of the displacement of hydrogen by a metal from an acid.
(b) Name two metals (other than zinc and iron) which can displace hydrogen from dilute hydrochloric acid ?
Solution :
(a) Magnesium reacts with very dilute nitric acid to form magnesium nitrate and hydrogen gas.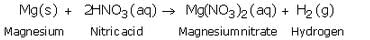
(b) Magnesium and aluminium.
Question 54:
What is the action of water on (a) sodium (b) magnesium, and (c) aluminium ? Write equations of the chemical reactions involved.
Solution :
(a) Sodium reacts vigorously with cold water forming sodium hydroxide and hydrogen gas.
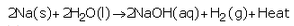
(b) Magnesium reacts with hot water to form magnesium hydroxide and hydrogen.
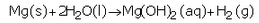
(c) Aluminium reacts with steam to form aluminium oxide and hydrogen gas.
Question 55:
You are given samples of three metals — sodium, magnesium and copper. Suggest any two activities to arrange them in order of their decreasing reactivities.
Solution :
(i) When sodium, magnesium and copper are left in air, sodium reacts vigorously with oxygen to form sodium oxide, magnesium reacts with oxygen to form magnesium oxide only on heating , whereas copper does not burn in air even on strong heating. It reacts only on prolonged heating. This shows that sodium is most reactive, then magnesium and copper is the least reactive among the three.
(ii) Sodium reacts vigorously with cold water to form sodium hydroxide and hydrogen, magnesium does not react with cold water but reacts with hot water to form magnesium hydroxide and hydrogen but copper does not react even with steam. This shows that sodium is highly reactive; magnesium is less reactive than sodium and copper is the least reactive among the three.
Question 56:
(a) Write one reaction in which aluminium oxide behaves as a basic oxide and another in which it behaves as an acidic oxide.
(b) What special name is given to substances like aluminium oxide.
(c) Name another metal oxide which behaves like aluminium oxide.
Solution :
(a)
In this reaction, aluminium oxide behaves as a basic oxide because it reacts with an acid to form salt and water.
In this reaction, aluminium oxide behaves as an acidic oxide because it reacts with a base to form salt and water.
(b) Amphoteric oxides.
(c) Zinc oxide.
Question 57:
(a) What happens when calcium reacts with water ? Write the chemical equation of the reaction of calcium with water.
(b) Write the chemical equation of the reaction which takes place when iron reacts with dilute sulphuric acid. What happens when the gas produced is ignited with a burning matchstick ?
Solution :
(a) Calcium reacts with cold water to form calcium hydroxide and hydrogen gas.
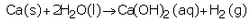
(b) When iron reacts with dilute sulphuric acid, it forms iron sulphate and hydrogen gas.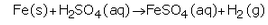
When hydrogen gas is ignited with a burning matchstick, it produces a ‘pop’sound.
Question 58:
You are given a dry cell, a torch bulb with holder, wires and crocodile clips. How would you use them to distinguish between samples of metals and non-metals ?
Solution :
We would create an apparatus using dry cell, a torch bulb fitted in a holder and some connecting wires with crocodile clips and connect them to make an electric circuit. Then insert a piece of sulphur between the crocodile clips and the bulb does not light up at all. This means that sulphur does not allow the electric current to pass through it. Now insert a piece of copper between the crocodile clips and the bulb will light up. This observation shows that non metals (ex- sulphur) do not conduct electricity and metals (ex- copper) conduct electricity.
Question 59:
State any five physical properties of metals and five physical properties of non-metals.
Solution :
Properties of metals:
(i) Metals are malleable i.e. they can be beaten into thin sheets with a hammer.
(ii) Metals are ductile i.e. they can be drawn into thin wires.
(iii) Metals are good conductors of heat and electricity.
(iv) Metals are lustrous.
(v) Metals are generally hard.
Properties of non-metals:
(i) Non-metals are non-malleable i.e. they cannot be beaten into thin sheets with a hammer.
(ii) Non-metals are non-ductile i.e. they cannot be drawn into thin wires.
(iii) Non-m etals are bad conductors of heat and electricity.
(iv) Non-m etals are non- lustrous.
(v) Non-m etals are generally soft .
Question 60:
(a) Name two physical properties each of sodium and carbon in which their behaviour is not as expected from their classification as metal and non-metal respectively.
(b) Name two metals whose melting points are so low that they melt when held in the hand.
Solution :
(a) Sodium metal: Soft, low melting point
Carbon non-metal: graphite conducts electricity; diamond has a very high melting point.
(b) Gallium and cesium.
Question 61:
Metals are said to be shiny. Why do metals generally appear to be dull ? How can their brightness be restored ?
Solution :
Metals lose their shine or brightness on keeping in air for a long time and acquire a dull appearence due to the formation of a thin layer of oxide, carbonate or sulphide on their surface by the slow action of various gases present in air.
Brightness of metals can be restored by rubbing the dull surface of the metal object with a sand paper, then the outer corroded layer is removed and the metal object becomes shiny and bright once again.
Question 62:
(a) What are metals ? Name five metals.
(b) Name a metal which is so soft that it can be cut with a knife.
(c) Name the metal which is the best conductor of heat and electricity.
(d) What happens when a metal reacts with dilute hydrochloric acid ? Explain with the help of an example.
(e) Write the equations for the reactions of :
(i) Magnesium with dilute hydrochloric acid
(ii) Aluminium with dilute hydrochloric acid
(iii) Zinc with dilute hydrochloric acid
(iv) Iron with dilute hydrochloric acid
Name the products formed in each case. Also indicate the physical states of all the substances involved.
Solution :
(a) Metals are the elements that conduct heat and electricity, and are malleable and ductile.
Example: Iron, aluminium, copper, gold and silver.
(b) Sodium
(c) Silver
(d) When a metal reacts with dilute hydrochloric acid, it forms metal chloride and hydrogen gas.
Example: Magnesium reacts rapidly with dilute hydrochloric acid to form magnesium chloride and hydrogen.
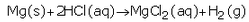
(e)(i)
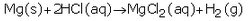
The products formed are magnesium chloride and hydrogen.
(ii)
The products formed are aluminium chloride and hydrogen.
(iii)
The products formed are zinc chloride and hydrogen.
(iv)
The products formed are iron chloride and hydrogen.
(Page No - 135)
Question 63:
(a) Define non-metals. Give five examples of non-metals.
(b) Name a non-metal which conducts electricity.
(c) Name a non-metal having lustre (shining surface).
(d) Name a non-metal which is extremely hard.
(e) How do non-metals react with oxygen ? Explain with an example. Give equation of the reaction involved. What is the nature of the product formed ? How will you demonstrate it ?
Solution :
(a)Non-metals are the elements that do not conduct heat and electricity and are neither malleable nor ductile.
Example: Carbon, sulphur, phosphorus, silicon and oxygen.
(b) Carbon.
(c) Iodine.
(d) Carbon (Diamond).
(e) Non-metals react with oxygen to form acidic oxides or neutral oxides. Carbon burns in air to form carbon dioxide.
C(s) + O2(g) --> CO2(g)
The nature of the product formed is acidic. When carbon dioxide dissolves in water, it forms carbonic acid. It turns blue litmus to red which shows it is acidic in nature.
Question 64:
(a) What is meant by the reactivity series of metals ? Arrange the following metals in an increasing order of their reactivities towards water :
Zinc, Iron, Magnesium, Sodium
(b) Hydrogen is not a metal but still it has been assigned a place in the reactivity series of metals. Why ?
(c) Name one metal more reactive and another less reactive than hydrogen.
(d) Name one metal which displaces copper from copper sulphate solution and one which does not.
(e) Name one metal which displaces silver from silver nitrate solution and one which does not.
Solution :
(a) The arrangement of metals in a vertical column in the order of decreasing reactivities is called reactivity series.
Increasing order of reactivity: Iron < zinc < magnesium < sodium
(b) Though hydrogen is not a metal but it has been placed in the reactivity series of metals due to the fact that like metals, hydrogen also loses electrons and forms positive ions.
(c) Lead is more reactive than hydrogen and copper is less reactive than hydrogen.
(d) Zinc displaces copper from copper sulphate solution and mercury does not displace copper from copper sulphate solution.
(e) Copper displaces silver from silver nitrate solution and gold does not.
Question 65:
(a) State any three differences between the physical properties of metals and non-metals.
(b) Differentiate between metals and non-metals on the basis of their chemical properties.
(c) State three reasons (of which at least one must be chemical) for believing that sodium is a metal.
(d) State three reasons (of which at least one must be chemical) for believing that sulphur is a non-metal.
(e) Which non-metal has been placed in the reactivity series of metals ?
Solution :
(a) Difference between metals and non-metals:
Metals
(i) Metals are malleable i.e. they can be beaten into thin sheets with a hammer.
(ii) Metals are ductile i.e. they can be drawn into thin wires.
(iii) Metals are good conductors of heat and electricity.
Non-metals
(i) Non-metals are non-malleable i.e. they cannot be beaten into thin sheets with a hammer.
(ii) Non-metals are non-ductile i.e. they cannot be drawn into thin wires.
(iii) Non-metals are bad conductors of heat and electricity.
(b) Difference between metals and non-metals:
Metals
(i) Metals form basic oxides.
(ii) Metals displace hydrogen from water
(iii) Metals displace hydrogen from dilute acids.
Non-metals
(i) Non-metals form acidic or neutral oxides.
(ii) Non-metals do not react with water.
(iii) Non-metals do not react with dilute acids.
(c) Sodium is a solid, it conducts electricity and forms basic oxides.
(d) Sulphur is a non-metal as it is brittle, non-ductile, non-conductor of electricity and forms acidic oxides.
(e) Hydrogen.
(Page No - 136)
Question 89:
An element E forms an oxide E2 An aqueous solution of E2O turns red litmus paper blue.
(a) What is the nature of the oxide E2O ?
(b) State whether element E is a metal or a non-metal.
(a) Give one example of an element like E.
Solution :
(a) Basic oxide.
(b) Metal .
(c) Sodium, Na .
Question 90:
Metal A burns in air, on heating, to form an oxide A2O3 whereas another metal B burns in air only on strong heating to form an oxide BO. The two oxides A2O3 and BO can react with hydrochloric acid as well as sodium hydroxide solution to form the corresponding salts and water.
(a) What is the nature of oxide A2O3 ?
(b) What is the nature of oxide BO ?
(c) Name one metal like A.
(d) Name one metal like B.
Solution :
(a) Amphoteric oxide .
(b) Amphoteric oxide .
(c) Aluminium, Al.
(d) Zinc, Zn .
Question 91:
An element X forms two oxides XO and XO2. The oxide XO has no action on litmus solution but oxide X02turns litmus solution red.
(a) What is the nature of oxide XO ?
(b) What is the nature of oxide XO2 ?
(c) Would you call element X a metal or a non-metal ? Give reason for your choice.
(d) Can you give an example of element like X ?
Solution :
(a) Neutral oxide.
(b) Acidic o xide .
(c) X is non-metal because non-metals form acidic and basic oxide .
(d) Carbon, C .
Question 92:
State and explain the reactions, if any, of the following metals with a solution of copper sulphate :
(a) Gold (b) Copper (c) Zinc (d) Mercury
Solution :
(a) No displacement reaction will take place because go ld is less reactive than copper.
(b) No reaction will take place between copper and copper sulphate solution ; there is no reaction possible.
(c) Zinc displaces copper from copper sulphate solution to form zinc sulphate solution and copper metal because zinc is m ore reactive than copper .
(d) No displacement reaction will take place because mercury is less reactive than copper
Question 93:
(a) Give the names and formulae of one metal chloride and one non-metal chloride.
(b) State an important property in which these metal chloride and non-metal chloride differ.
(c) Why do they differ in this property ?
Solution :
Non-metal chloride:
Carbon tetrachloride, CCl 4
(b) Sodium chloride solution conducts electricity whereas carbon tetrachlori de does not conduct electricity.
(c) Sodium chloride is an ionic compound whereas carbon tetrachloride is a covalent compound.
(Page No - 137)
Question 94:
In a solution of lead acetate, a strip of metal M was dipped. After some time, lead from the solution was deposited on the metal strip. Which metal is more reactive, M or lead ?
Solution :
M is more reactive than lead since it is able to displace lead from lead acetate solution
Question 95:
CuSO4(aq) + Fe(s) -- > FeSO4(aq) + Cu(s)
FeSO4(aq) + Zn(s) -- > ZnSO4(aq) + Fe(s)
On the basis of the above reactions, indicate which is most reactive and which is least reactive metal out of zinc, copper and iron.
Solution :
Zinc is most reactive and copper is least reactive out of the three since iron displaced copper from its solution and zinc displaced iron from its solution.
Question 96:
Which of the following reactions will not occur ? Why not ?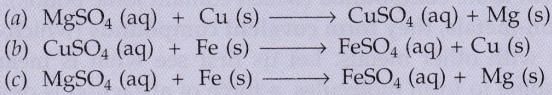
Solution :
Reaction (a) will not occur because Cu is less reactive than Mg
Reaction (c) will also not occur because Fe is less reactive than Mg .
Question 97:
In nature, metal A is found in a free state while metal B is found in the form of its compounds. Which of these two will be nearer to the top of the activity series of metals ?
Solution :
Metal B will be nearer to the top of the activity series since it is highly reactive and is hence found in the form of its compounds and not in free state.
Question 98:
If A, B, C, D, E, F, G, H, I, J and K represent metals in the decreasing order of their reactivity, which one of them is most likely to occur in a free state in nature ?
Solution :
K being the lowest in the reactivity series is least reactive and is most likely to occur in a free state in nature.
Question 99:
(a) Name a metal for each case :
(i) It does not react with cold as well as hot water but reacts with steam.
(ii) It does not react with any physical state of water.
(b) When calcium metal is added to water, the gas evolved does not catch fire but the same gas evolved on adding sodium metal to water catches fire. Why is it so ?
Solution :
(a) (i) Iron (ii) Gold
(b) More heat is evolved during the reaction of sodium metal with water due to which the hyd rogen gas formed catches fire. O n the other hand, less heat is evolved during the reaction of calcium metal with water which cannot make the hydrogen gas bu rn .
Question 100:
A zinc plate was kept in a glass container having CuS04 On examining it was found that the blue colour of the solution is getting lighter and lighter. After a few days, when the zinc plate was taken out of the solution, a number of small holes were noticed in it. State the reason and give chemical equation of the reaction involved.
Solution :
Zinc metal is more reactive than copper. Some of the zinc metal of zinc plate dissolves and displaces copper from copper sulphate solution. This dissolving of zinc metal forms tiny holes in zinc plate. Blue colour of copper sulphate solution gets lighter and lighter due to the formation of colourless zinc sulphate solution.
|
80 videos|569 docs|80 tests
|
FAQs on Lakhmir Singh & Manjit Kaur: Metals and Non-metals, Solutions- 2 - Science Class 10
| 1. What are some examples of metals and non-metals? |  |
| 2. How can metals and non-metals be distinguished from each other? |  |
| 3. What are the properties of metals? |  |
| 4. What are the properties of non-metals? |  |
| 5. Can an element have properties of both metals and non-metals? |  |





















