NEET Exam > NEET Notes > Chemistry Class 12 > Mind Map: Chemical Kinetics
Mind Map: Chemical Kinetics | Chemistry Class 12 - NEET PDF Download
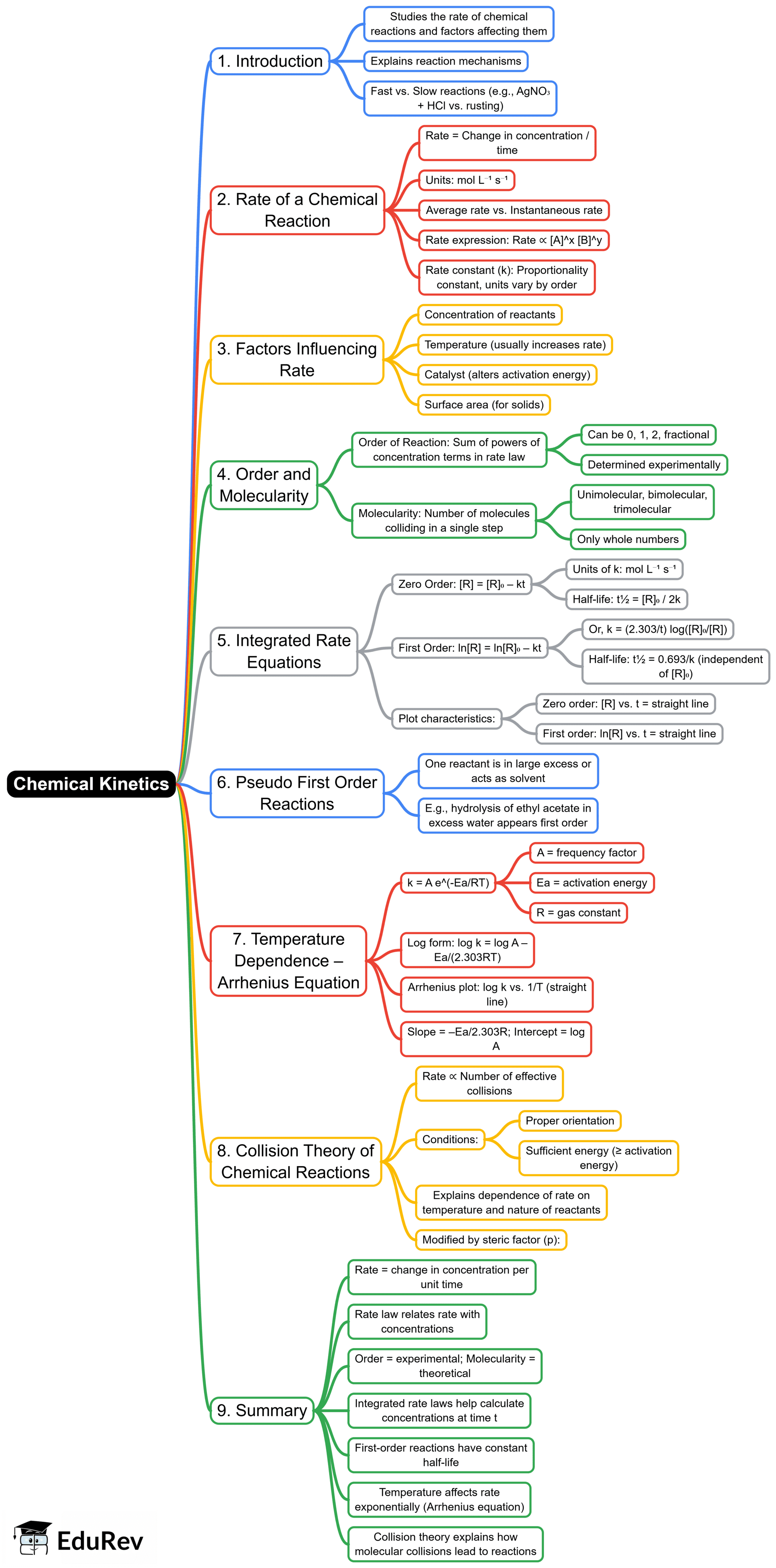
The document Mind Map: Chemical Kinetics | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|349 docs|78 tests
|
FAQs on Mind Map: Chemical Kinetics - Chemistry Class 12 - NEET
| 1. What is chemical kinetics and why is it important in chemistry? |  |
Ans.Chemical kinetics is the branch of chemistry that deals with the study of reaction rates and the factors affecting them. It is important because it helps in understanding how quickly reactions occur, the mechanisms behind these reactions, and how different conditions (like temperature and concentration) can influence the speed of a reaction. This knowledge is crucial for applications in fields such as pharmaceuticals, environmental science, and industrial processes.
| 2. What factors affect the rate of a chemical reaction? |  |
Ans.The rate of a chemical reaction can be affected by several factors including concentration of reactants, temperature, surface area of solid reactants, presence of catalysts, and the nature of the reactants. For instance, increasing the concentration of reactants often leads to a higher rate of reaction because there are more particles available to collide and react. Similarly, higher temperatures generally increase reaction rates by providing more energy for collisions.
| 3. How is reaction order determined, and what does it indicate about a reaction? |  |
Ans.Reaction order is determined by analyzing the rate law of a reaction, which expresses the rate of a chemical reaction as a function of the concentration of reactants. The order indicates how the rate is affected by the concentration of each reactant. A reaction can be zero-order, first-order, or second-order based on the exponent of the concentration term in the rate equation. For example, in a first-order reaction, doubling the concentration of the reactant will double the reaction rate.
| 4. What is the role of catalysts in chemical kinetics? |  |
Ans.Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy, allowing more reactant molecules to collide with enough energy to react. This can significantly speed up reactions and is widely used in industrial processes to enhance efficiency and reduce energy costs.
| 5. What is the Arrhenius equation, and how does it relate to chemical kinetics? |  |
Ans.The Arrhenius equation describes the temperature dependence of reaction rates. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the frequency factor, Ea is the activation energy, R is the universal gas constant, and T is the temperature in Kelvin. This equation highlights the importance of temperature and activation energy in determining how quickly a reaction will occur, making it a fundamental concept in chemical kinetics.
Related Searches
















