UPSC Exam > UPSC Notes > Class 6 to 12 NCERT Mindmaps for UPSC Preparation > Mindmap: Combustion and Flames
Mindmap: Combustion and Flames | Class 6 to 12 NCERT Mindmaps for UPSC Preparation PDF Download
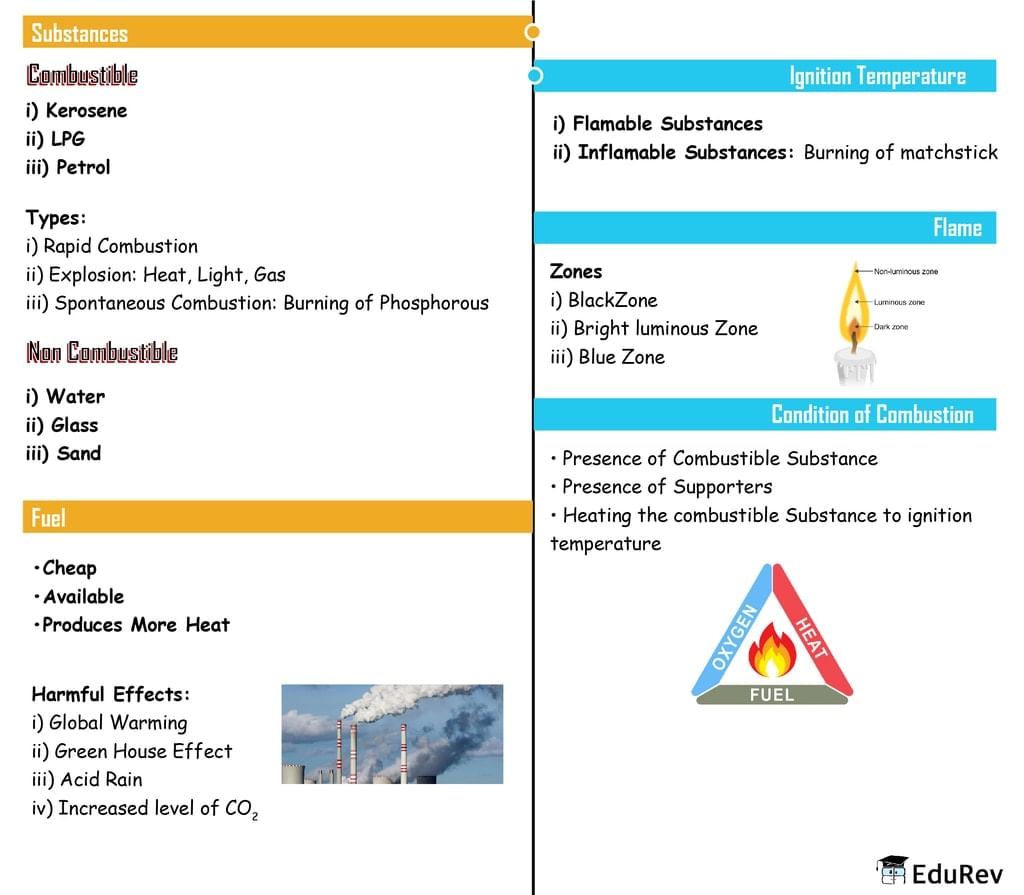
The document Mindmap: Combustion and Flames | Class 6 to 12 NCERT Mindmaps for UPSC Preparation is a part of the UPSC Course Class 6 to 12 NCERT Mindmaps for UPSC Preparation.
All you need of UPSC at this link: UPSC
FAQs on Mindmap: Combustion and Flames - Class 6 to 12 NCERT Mindmaps for UPSC Preparation
| 1. What is combustion and how does it occur? |  |
Ans. Combustion is a chemical process in which a substance reacts with oxygen to produce heat and light. It occurs when a fuel source combines with oxygen in the presence of an ignition source, such as heat or a spark. This reaction releases energy in the form of heat and light.
| 2. What are the different types of flames? |  |
Ans. Flames can be categorized into three types: diffusion flames, premixed flames, and laminar flames. Diffusion flames occur when a fuel source and oxygen mix in a non-uniform manner, resulting in a yellow, flickering flame. Premixed flames occur when the fuel and oxygen mix uniformly before combustion, producing a blue flame. Laminar flames refer to flames that burn smoothly and steadily, without turbulence.
| 3. How does combustion contribute to air pollution? |  |
Ans. Combustion releases various pollutants into the atmosphere, contributing to air pollution. When fuels burn, they release carbon dioxide (CO2), a greenhouse gas that contributes to climate change. Additionally, combustion can produce nitrogen oxides (NOx) and sulfur dioxide (SO2), which contribute to the formation of smog and acid rain. Incomplete combustion can also release carbon monoxide (CO) and particulate matter, which can be harmful to human health.
| 4. What are some examples of combustion reactions? |  |
Ans. Combustion reactions occur in various everyday scenarios. Some examples include the burning of gasoline in car engines, the combustion of natural gas for heating and cooking, and the burning of wood in fireplaces. Combustion reactions also occur in industrial processes, such as power generation in thermal power plants and the burning of fossil fuels for energy production.
| 5. How can combustion be controlled to improve efficiency? |  |
Ans. Combustion efficiency can be improved by controlling the air-fuel ratio, ensuring sufficient oxygen supply for complete combustion. Proper ventilation and insulation can reduce heat loss and improve efficiency. Additionally, using advanced combustion technologies, such as catalytic converters and clean-burning fuels, can help reduce emissions and improve overall combustion efficiency.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches

















