UPSC Exam > UPSC Notes > Class 6 to 12 NCERT Mindmaps for UPSC Preparation > Mindmap: Periodic Classification of Elements
Mindmap: Periodic Classification of Elements | Class 6 to 12 NCERT Mindmaps for UPSC Preparation PDF Download
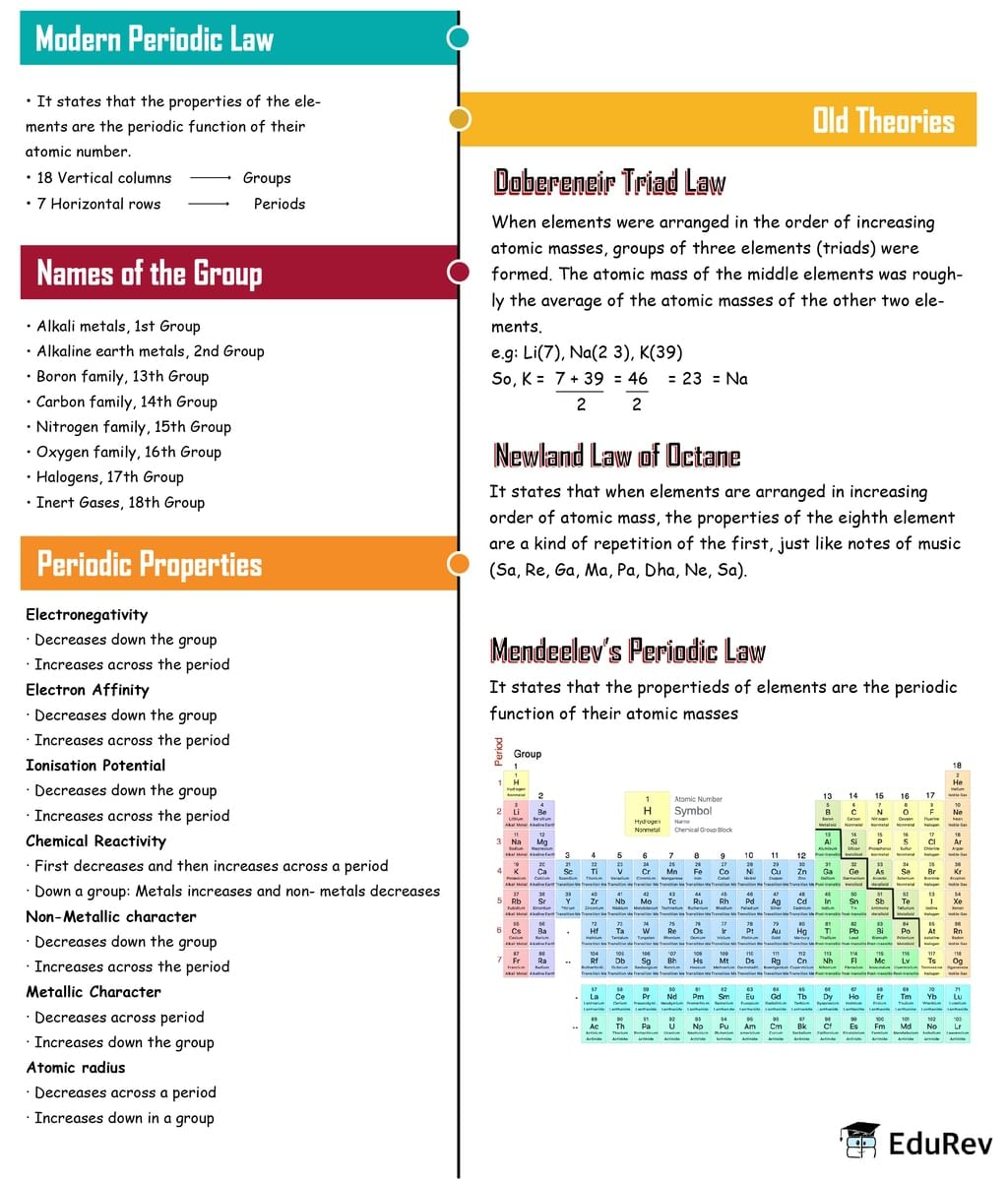
The document Mindmap: Periodic Classification of Elements | Class 6 to 12 NCERT Mindmaps for UPSC Preparation is a part of the UPSC Course Class 6 to 12 NCERT Mindmaps for UPSC Preparation.
All you need of UPSC at this link: UPSC
FAQs on Mindmap: Periodic Classification of Elements - Class 6 to 12 NCERT Mindmaps for UPSC Preparation
| 1. What is periodic classification of elements? |  |
Ans. Periodic classification of elements is the arrangement of elements in a systematic manner based on their similar properties and increasing atomic number. It helps to organize the numerous elements into periods (rows) and groups (columns) in a way that reflects their chemical behavior and trends.
| 2. What is the significance of periodic classification of elements? |  |
Ans. The periodic classification of elements is significant because it allows us to understand the relationships between different elements and predict their properties. It helps in identifying trends such as atomic size, valency, electronegativity, and chemical reactivity. This classification also provides a basis for the study of periodicity and the development of the periodic table.
| 3. How are elements arranged in the periodic table? |  |
Ans. Elements in the periodic table are arranged in order of increasing atomic number. They are organized into periods (rows) and groups (columns) based on their similar properties. Each period represents a new energy level or shell, while the groups consist of elements with similar electronic configurations and chemical behaviors.
| 4. What are the main features of the modern periodic table? |  |
Ans. The main features of the modern periodic table are:
- Elements are arranged in order of increasing atomic number.
- Elements are organized into periods and groups.
- The periods represent the energy levels or shells of atoms.
- The groups consist of elements with similar electronic configurations.
- The periodic table is divided into s, p, d, and f blocks based on the subshells being filled.
- The elements in each group exhibit similar chemical properties and trends.
| 5. How does the periodic table help in predicting the properties of elements? |  |
Ans. The periodic table helps in predicting the properties of elements by identifying trends and patterns. Elements in the same group tend to have similar properties due to their similar electronic configurations. For example, elements in Group 1 (alkali metals) have a tendency to lose one electron and form +1 ions. Similarly, elements in Group 17 (halogens) have a tendency to gain one electron and form -1 ions. By understanding these trends, we can make predictions about the reactivity, valency, and other properties of elements based on their position in the periodic table.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches

















