Mnemonics: Metals and Non-Metals | Science Class 10 PDF Download
| Table of contents |

|
| 1. Physical Properties for Metals |

|
| 2. Physical Properties of Non-Metals |

|
| 3. Chemical Properties of Metals |

|
| 4. Metal Reactivity Series |

|
| 5. Extraction of Metals (Activity Series based) |

|
1. Physical Properties for Metals
Mnemonic: Lovely Heavy Ducks Make Great Sounds Daily Morning Beeps or LHD-MD-SDM.
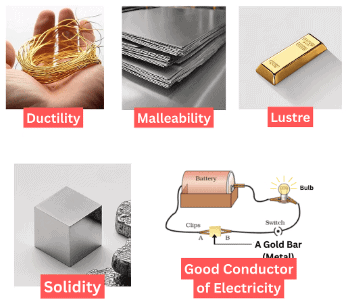
Mnemonic Explanation:
- Lovely – Lustrous
- Heavy – Hard
- Ducks – Ductile
- Make– Malleable
- Great – Good conductor of heat and electricity
- Sounds – Sonorous
- Daily – Dense
- Morning Beeps– High Melting & Boiling Point
2. Physical Properties of Non-Metals
Mnemonic: Dull Penguins Do Break Like Melting & Boiling Soap
Mnemonic Explanation: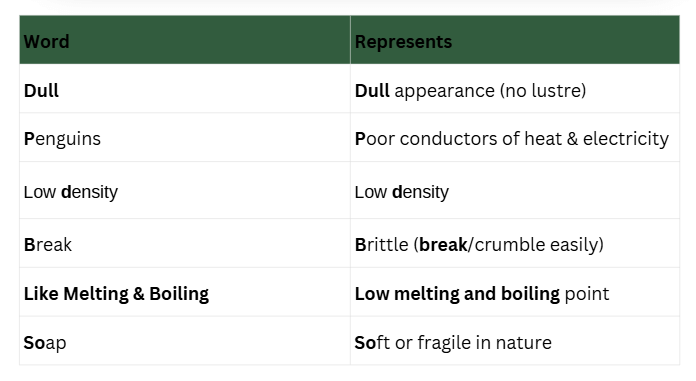
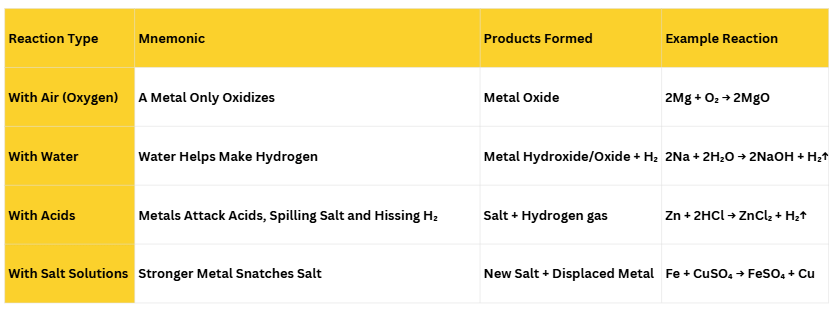
3. Chemical Properties of Metals
Mnemonic :
Mnemonic Explanation:
(i) Metals react with air to form Metal oxides.
(ii) Metal reacts with water ( Cold, Hot, Steam ) to form Metal Hydroxide/ Oxide and Hydrogen.
- Metals like potassium and sodium react violently with cold water.
- Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
- Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen.
- Metals like aluminium, iron and zinc do not react either with cold or hot water. However, they react with steam to form metal oxides and hydrogen.
(iii) Metals react with Acid to form salt and hydrogen gas.
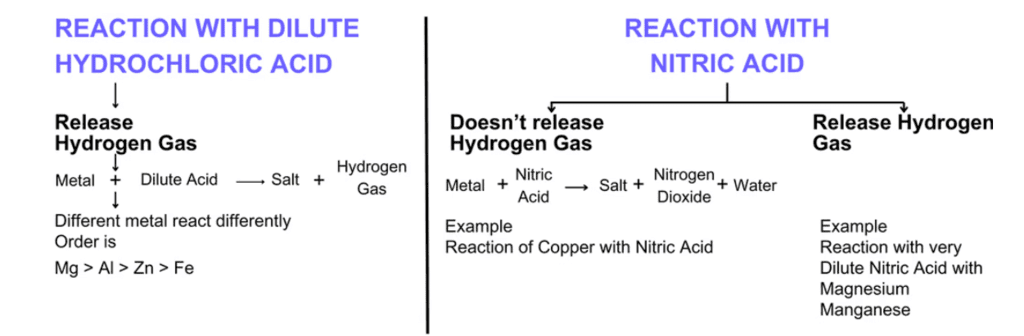
Note: Cu, Ag, Hg don’t react with dilute acids
(iv) Metal reacts with salt with a stronger metal salt and displaces a weaker metal alone.
4. Metal Reactivity Series
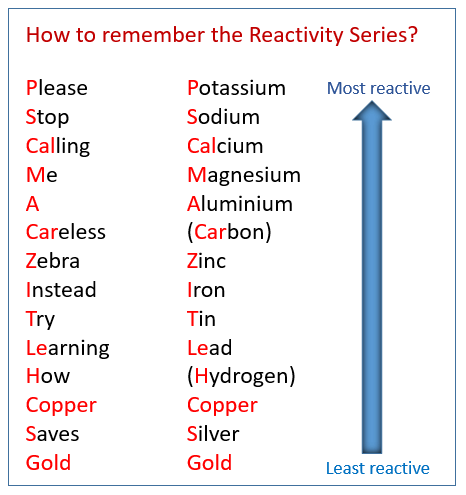
Mnemonic :- Please Stop Calling Me A Careless Zebra Instead Try Learning How Copper Saves Gold.
Mnemonic Explanation:
5. Extraction of Metals (Activity Series based)
Mnemonic: Low metals: "Some Cats Prefer Selfies"
- Some – Sulphide ores
- Cats – Converted to oxides
- Prefer – Partial oxidation
- Selfies – Self-reduction
Mnemonic: Middle metals: "Roasted Cakes Reduce Cravings"
- Roasting (for sulphides)
- Calcination (for carbonates)
- Reduction with carbon
- Cravings = Crude metal
Mnemonic: Top metals: "Electrolytes Can Solve Problems"
- Electrolytes – Electrolytic reduction
- Can – Cathode
- Solve – Sodium
- Problems – Potassium, etc.
Mnemonic Explanation:
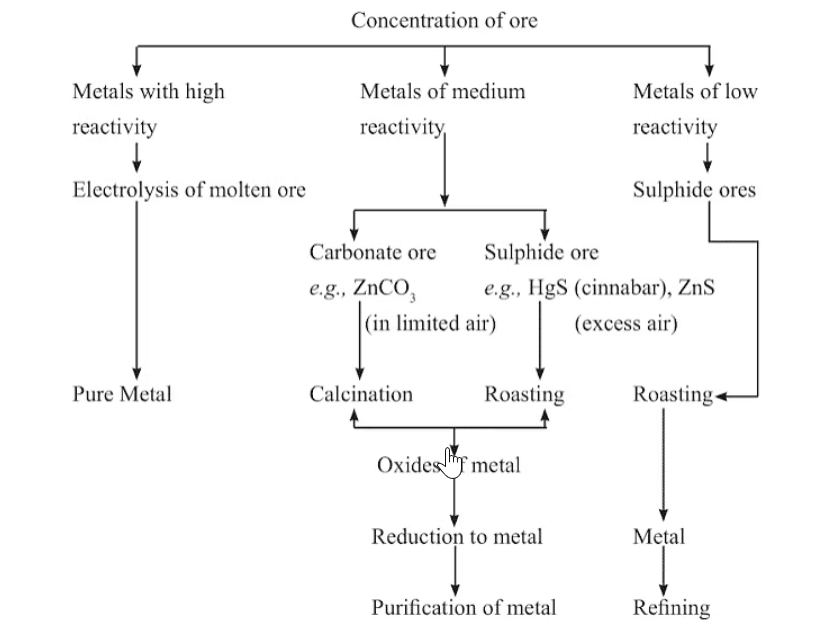
These mnemonics can help make it easier to recall the elements in each category!
|
80 videos|567 docs|80 tests
|
FAQs on Mnemonics: Metals and Non-Metals - Science Class 10
| 1. What are the key physical properties of metals? |  |
| 2. How do non-metals differ in their physical properties compared to metals? |  |
| 3. What are the main chemical properties of metals? |  |
| 4. What is the metal reactivity series and why is it important? |  |
| 5. How can a mnemonic help in remembering the extraction of metals based on their activity series? |  |