Mnemonics: Structure of Atom | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| Introduction |

|
| Principal Quantum Number (n) |

|
| Azimuthal Quantum Number (l) |

|
| Magnetic Quantum Number (mₗ) |

|
| Spin Quantum Number (mₛ) |

|
| Aufbau Principle |

|
| Pauli Exclusion Principle |

|
| Hund’s Rule |

|
Introduction
Get ready to simplify your study of quantum mechanics! These mnemonics will make understanding quantum numbers and atomic principles both fun and easy to remember.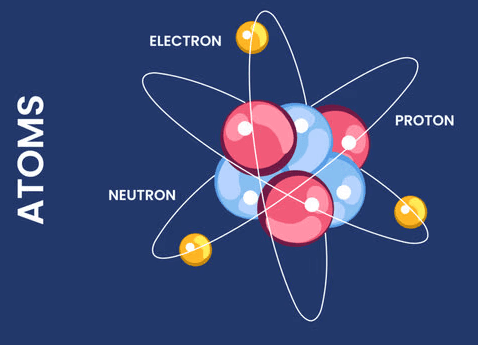
Principal Quantum Number (n)
Describes: The energy level or shell where the electron is located.
- Mnemonic: "Principal Never Leaves School."
- Principal : Principal Quantum Number
- Never: Number denoted by (n)
- School: Indicates the electron shell.
This mnemonic emphasizes that the Principal Quantum Number specifies the energy level or shell of the electron. 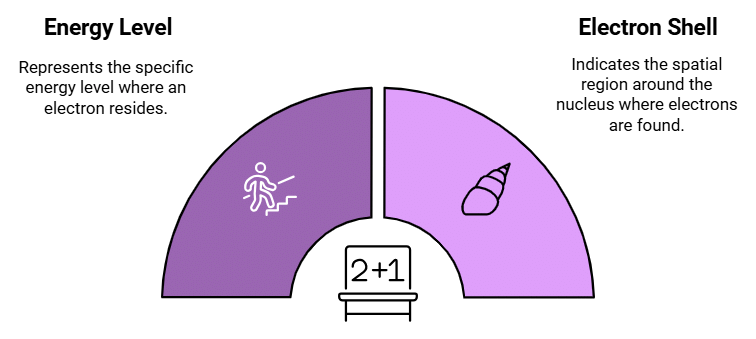
Azimuthal Quantum Number (l)
Describes: The shape of the orbital (s, p, d, f).
- Mnemonic: "Artists Love Shapes."
- Artists: Azimuthal Quantum Number
- Love: Denoted by (l)
- Shapes: Represents the Shapes of the orbital.
This mnemonic links the Azimuthal Quantum Number with the concept of orbital shape, such as spherical (s), dumbbell-shaped (p), etc.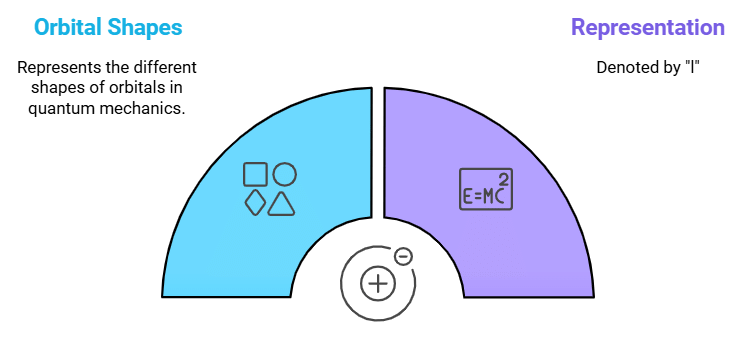
Magnetic Quantum Number (mₗ)
Describes: The orientation of the orbital in space.
- Mnemonic: "Magnetic Moments Locate Orbits."
- Magnetic : Magnetic Quantum Number
- Moments : Denoted by (m1)
- Orbital : Denotes the Magnetic moment or orientation of the orbital
This mnemonic highlights how the Magnetic Quantum Number specifies the orbital orientation around the nucleus.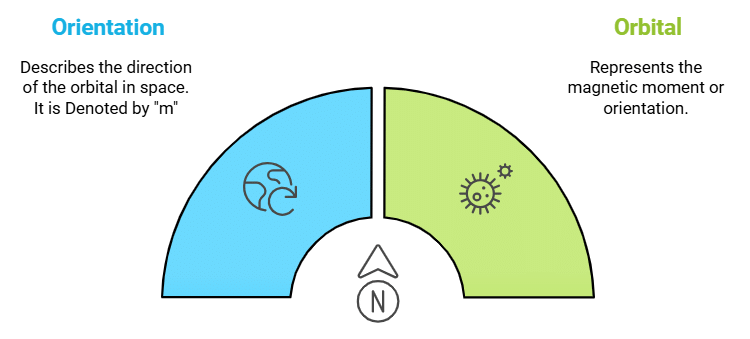
Spin Quantum Number (mₛ)
Describes: The spin of the electron (clockwise or counterclockwise).
- Mnemonic: "Spinning Means Switching."
- Spinning : Spin
- Means : Means
- Switching : Switching (clockwise or counterclockwise, +½ or −½)
This mnemonic underscores that the Spin Quantum Number describes whether the electron is spinning up or down.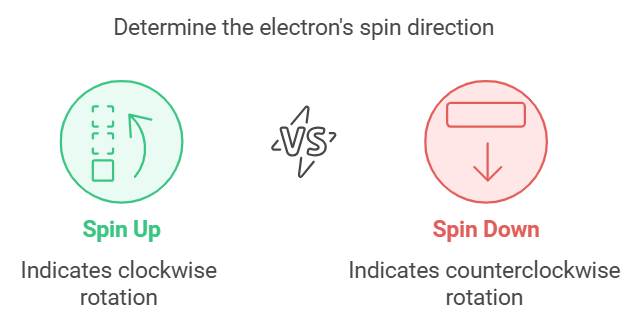
Aufbau Principle
Statement: The Aufbau Principle states that electrons fill atomic orbitals in order of increasing energy levels, starting with the lowest energy orbital (e.g., 1s) and moving to higher ones (e.g., 2s, 2p, 3s, etc.).
- Mnemonics: "Some Pretty Dogs Fetch Sticks."
- Some : s-orbitals (lowest energy, spherical shape).
- Pretty : p-orbitals (next in energy, dumbbell shape).
- Dogs : d-orbitals (higher energy, clover shape).
- Fetch : f-orbitals (highest energy, complex shape).
This mnemonic outlines the order of orbitals (s, p, d, f) as they appear in the Aufbau sequence.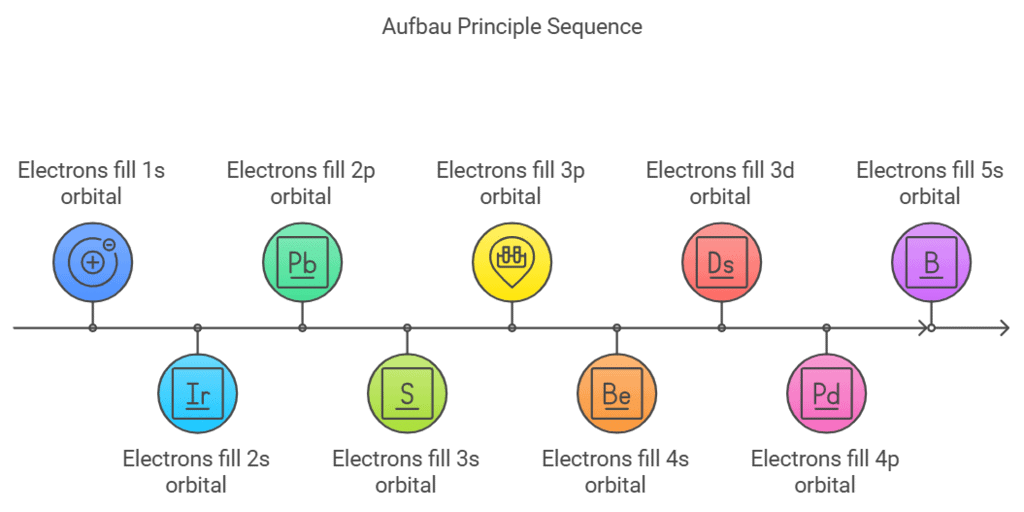
Pauli Exclusion Principle
Rule: No two electrons in the same atom can have identical quantum numbers.
Mnemonic: "Pauli Prohibits Twin IDs."
Hund’s Rule
Rule: Electrons occupy orbitals singly before doubling up in the same orbital.
Mnemonic: "Hund Hands Out Single Seats."
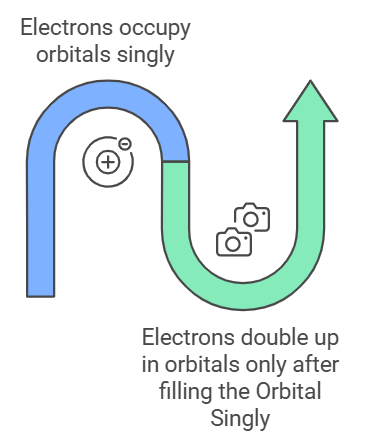
With these mnemonics, you'll have a clearer understanding of quantum theory, making it easier to remember and apply these important scientific principles.
|
114 videos|263 docs|74 tests
|
















