Mnemonics: Coordination Compounds | Chemistry Class 12 - NEET PDF Download
Coordination compounds may seem complex, but with the help of these engaging mnemonics, you’ll master their structures, naming rules, and bonding principles in no time! Let's make learning fun and memorable.
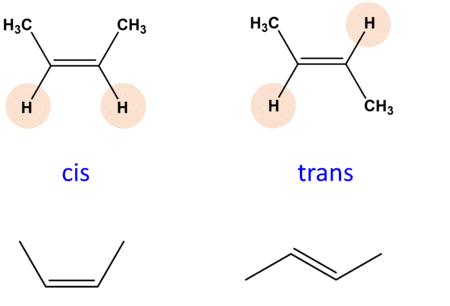
Geometrical Isomerism in Coordination Compounds
- Cis isomer: Ligands are positioned next to each other, usually at a 90° or 180° angle.
- Trans isomer: Ligands are positioned opposite each other, typically at 180°.
Mnemonic: "Cis Stays Close, Trans Travels Away."
- Cis Stays Close: In Cis isomerism, Similar ligands stay close (90° angle)
- Trans Travels Away: In Trans isomerism, Similar ligands are opposite (180° angle)
Common Ligands & Their Formulas
Mnemonic: "Strong Cats Never Find Cool Orchids"
- S – SCN⁻ (thiocyanato)
- C – Cl⁻ (chloro)
- N – NH₃ (ammine)
- F – F⁻ (fluoro)
- C – CN⁻ (cyano)
- O – OH⁻ (hydroxo)
Order of Naming Ligands
Naming goes: Negative ligands → Neutral ligands → Positive ligands (alphabetical within each group).
Mnemonic: "Negative Neighbours Need Positive People"
Common Neutral Ligands
- NH₃ – ammine
- H₂O – aqua
- CO – carbonyl
- NO – nitrosyl
Mnemonic: "Aunt Amy's Aqua Carbonated Noodles"
Oxidation Number Calculation
The rule: Oxidation number = (Charge on complex) – (Sum of ligand charges)
Mnemonic: "Complex Charge Comes Last" (First sum ligands, then subtract from total charge).
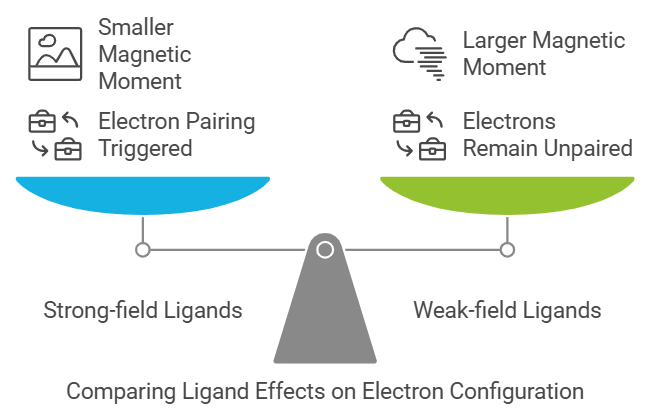
Crystal Field Theory
Explains: The difference between strong-field and weak-field ligands.
Mnemonic: "Strong Tigers Love Low Energy."
- Strong: Strong-field ligands (e.g., CN⁻, CO)
- Tigers: Trigger pairing of electrons (low spin)
- Love: Leads to
- Low Energy: Smaller magnetic moment and lower energy configuration
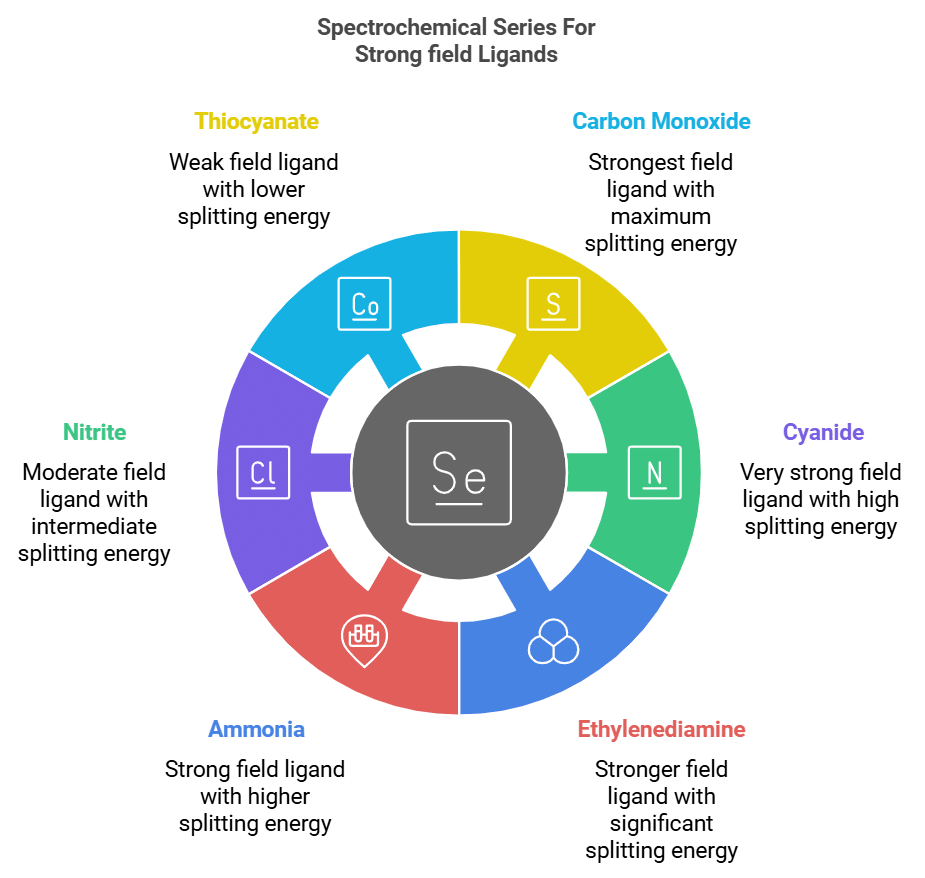
Spectrochemical Series: Strong Field Ligand
The ligands in increasing order of their field strength are:
NCS⁻ (Thiocyanate), NO₂⁻ (Nitrite), NH₃ (Ammonia), en (Ethylenediamine), CN⁻ (Cyanide), CO (Carbon Monoxide).
Mnemonic : "Nancy's Naughty Nephew Eats Chocolate Cake."
- Nancy's: NCS⁻ (Thiocyanate)
- Naughty: NO₂⁻ (Nitrite)
- Nephew: NH₃ (Ammonia)
- Eats: en (Ethylenediamine)
- Chocolate: CN⁻ (Cyanide)
- Cake: CO (Carbon Monoxide)
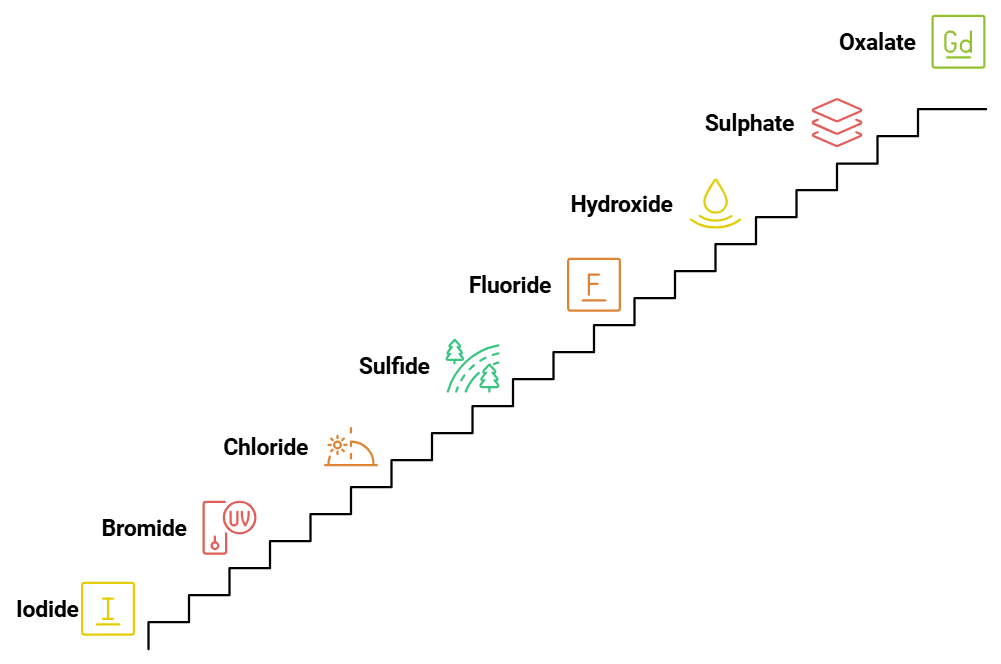
Spectrochemical Series: Weak Field Ligand
The weak field ligands in increasing order of their field strength are:
I⁻ (Iodide) < Br⁻ (Bromide) < Cl⁻ (Chloride) < S²⁻ (Sulfide) < F⁻ (Fluoride) < OH⁻ (Hydroxide) < SO₄²⁻ (Sulphate) < C₂O₄²⁻ (Oxalate) < H₂O (Water).
Mnemonic: "I Bring Clear Smiles, For Our Sweet Children Who Wait."
- I: Iodide (I⁻)
- Bring: Bromide (Br⁻)
- Clear: Chloride (Cl⁻)
- Smiles: Sulfide (S²⁻)
- For: Fluoride (F⁻)
- Our: Hydroxide (OH⁻)
- Sweet: Sulphate (SO₄²⁻)
- Children: Oxalate (C₂O₄²⁻)
- Who: Water (H₂O)
- Wait: (as a reminder, indicating they are the weaker field ligands)
Weak field ligands → small crystal field splitting (Δ) → often high-spin
Strong field ligands → large crystal field splitting (Δ) → often low-spin
Order from weakest to strongest field ligand:
I⁻ < Br⁻ < Cl⁻ < F⁻ < OH⁻ < H₂O < NH₃ < en < NO₂⁻ < CN⁻ < CO
Quick tip:
- Strong field ligands (like CN⁻, CO) → low spin, pairing occurs before filling higher orbitals.
- Weak field ligands (like I⁻, Br⁻) → high spin, electrons occupy higher orbitals before pairing.
|
75 videos|278 docs|78 tests
|
FAQs on Mnemonics: Coordination Compounds - Chemistry Class 12 - NEET
| 1. What is geometrical isomerism in coordination compounds? |  |
| 2. How does Crystal Field Theory explain the color of coordination compounds? |  |
| 3. What are strong field and weak field ligands in the context of the spectrochemical series? |  |
| 4. How can mnemonics help in remembering the order of ligands in the spectrochemical series? |  |
| 5. Why is the understanding of geometrical isomerism important in coordination chemistry? |  |

















