NCERT Based Activity: Carbon and its Compounds | Science Class 10 PDF Download
Activity 4.1: Identifying Carbon-Based Materials
- Make a list of ten things you have used or consumed since the morning.
- Compile this list with the lists made by your classmates and then sort the items into the adjacent table.
- If there are items which are made up of more than one material, put them into both the relevant columns of the table.
- Look at the items that come in the last column of the above table filled by you – your teacher will be able to tell you that most of them are made up of compounds of carbon. Can you think of a method to test this? What would be the product if a compound containing carbon is burnt? Do you know of any test to confirm this?
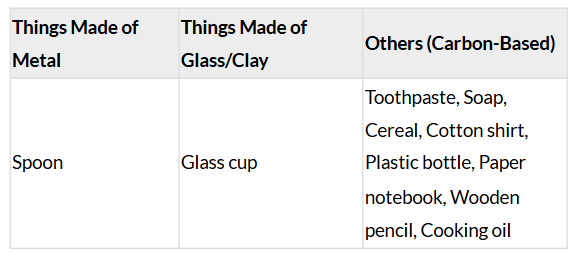
Ans: Sample List of Items (Examples):
- Toothpaste, 2. Soap, 3. Breakfast cereal, 4. Cotton shirt, 5. Plastic water bottle, 6. Glass cup, 7. Metal spoon, 8. Paper notebook, 9. Wooden pencil, 10. Cooking oil.
Table Sorting Items:
Testing for Carbon Compounds:
- Method: Burn a small sample of the item (e.g., paper, cotton, or plastic) in a controlled environment using a flame.
- Product of Burning: If the compound contains carbon, it will produce carbon dioxide (CO₂) and possibly soot (carbon) or water (H₂O) depending on the composition. Incomplete combustion may produce carbon monoxide (CO) and soot.
- Confirmation Test for CO₂: Pass the gas produced during burning through lime water (calcium hydroxide solution, Ca(OH)₂). If CO₂ is present, the lime water turns milky due to the formation of calcium carbonate (CaCO₃).
- Reaction: Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
Conclusion: Most items in the “Others” column (e.g., paper, cotton, plastic) are carbon-based, as confirmed by the production of CO₂ upon burning.
Activity 4.2: Analyzing Homologous Series of Carbon Compounds
- Calculate the difference in the formulae and molecular masses for (a) CH₃OH and C₂H₅OH, (b) C₂H₅OH and C₃H₇OH, and (c) C₃H₇OH and C₄H₉OH.
- Is there any similarity in these three?
- Arrange these alcohols in the order of increasing carbon atoms to get a family. Can we call this family a homologous series?
- Generate the homologous series for compounds containing up to four carbons for the other functional groups given in Table 4.3.
Ans: Difference in Formulae and Molecular Masses:
(a) CH₃OH and C₂H₅OH:
- Formula Difference: C₂H₅OH – CH₃OH = C₂H₅OH – CH₃OH = CH₂ (one additional CH₂ unit).
- Molecular Mass:
- CH₃OH: C = 12, H = 1×4, O = 16 → 12 + 4 + 16 = 32 u.
- C₂H₅OH: C = 12×2, H = 1×6, O = 16 → 24 + 6 + 16 = 46 u.
- Difference: 46 – 32 = 14 u (mass of CH₂ = 12 + 2 = 14 u).
(b) C₂H₅OH and C₃H₇OH:
- Formula Difference: C₃H₇OH – C₂H₅OH = CH₂.
- Molecular Mass:
- C₂H₅OH: 46 u (as above).
- C₃H₇OH: C = 12×3, H = 1×8, O = 16 → 36 + 8 + 16 = 60 u.
- Difference: 60 – 46 = 14 u.
(c) C₃H₇OH and C₄H₉OH:
- Formula Difference: C₄H₉OH – C₃H₇OH = CH₂.
- Molecular Mass:
- C₃H₇OH: 60 u (as above).
- C₄H₉OH: C = 12×4, H = 1×10, O = 16 → 48 + 10 + 16 = 74 u.
- Difference: 74 – 60 = 14 u.
Similarity:
- Each pair differs by a CH₂ unit in their molecular formula, and the molecular mass difference is consistently 14 u.
- All compounds contain the same functional group (–OH, alcohol).
Arranging Alcohols and Homologous Series:
- Order (Increasing Carbon Atoms): CH₃OH (methanol, 1C), C₂H₅OH (ethanol, 2C), C₃H₇OH (propanol, 3C), C₄H₉OH (butanol, 4C).
Homologous Series: Yes, this is a homologous series because:
- Each successive member differs by a CH₂ unit.
- They have the same functional group (–OH).
- They share similar chemical properties and show a gradation in physical properties (e.g., increasing boiling points).
4. Homologous Series for Other Functional Groups (Up to 4 Carbons, Table 4.3):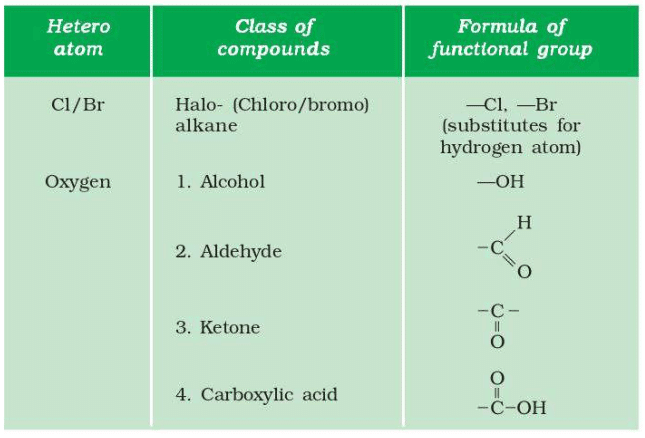
- Haloalkanes (e.g., Chloroalkane, –Cl):
- 1C: CH₃Cl (chloromethane).
- 2C: C₂H₅Cl (chloroethane).
- 3C: C₃H₇Cl (chloropropane).
- 4C: C₄H₉Cl (chlorobutane).
- Aldehydes (–CHO):
- 1C: HCHO (methanal).
- 2C: CH₃CHO (ethanal).
- 3C: C₂H₅CHO (propanal).
- 4C: C₃H₇CHO (butanal).
- Ketones (–CO–):
- 3C: CH₃COCH₃ (propanone).
- 4C: CH₃COCH₂CH₃ (butanone).
- Carboxylic Acids (–COOH):
- 1C: HCOOH (methanoic acid).
- 2C: CH₃COOH (ethanoic acid).
- 3C: C₂H₅COOH (propanoic acid).
- 4C: C₃H₇COOH (butanoic acid).
Conclusion: The alcohols form a homologous series, and similar series can be generated for other functional groups, each differing by a CH₂ unit and sharing the same functional group.
Activity 4.3: Combustion of Carbon Compounds
- CAUTION: This Activity needs the teacher's assistance. Take some carbon compounds (naphthalene, camphor, alcohol) one by one on a spatula and burn them.
- Observe the nature of the flame and note whether smoke is produced. Place a metal plate above the flame.
- Is there a deposition on the plate in case of any of the compounds?
Observations: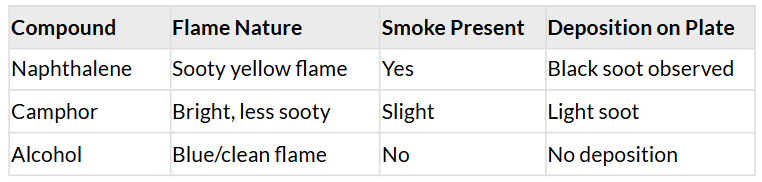
Conclusion:
- Naphthalene and camphor, being solid carbon-rich compounds, burn with sooty flames, indicating incomplete combustion.
- Alcohol, a cleaner-burning fuel, burns with a blue flame and no smoke, showing complete combustion.
- The black deposition on the metal plate is carbon soot, which is a common byproduct of incomplete combustion.
Concept Demonstrated: This activity illustrates how different carbon compounds burn differently based on their composition. It also shows the difference between complete and incomplete combustion, as well as the production of soot (carbon particles).
Activity 4.4: Observing Flame Types in a Bunsen Burner
- Light a bunsen burner and adjust the air hole at the base to get different types of flames/presence of smoke.
Observations: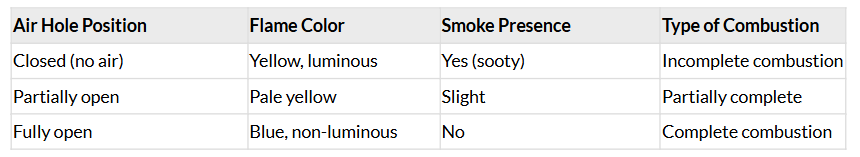
Q: When do you get a yellow, sooty flame?
Ans: When the air hole is closed or only slightly open. This leads to incomplete combustion due to insufficient oxygen.
Q: When do you get a blue flame?
Ans: When the air hole is fully open, allowing maximum air (oxygen) to mix with the gas, resulting in complete combustion.
Conclusion: The amount of air (oxygen) mixed with the gas determines the type of flame. A yellow, sooty flame indicates incomplete combustion and releases carbon particles (soot). A blue flame indicates complete combustion, which is hotter and cleaner.
Activity 4.5: Oxidation of Ethanol
- Take about 3 mL of ethanol in a test tube and warm it gently in a water bath.
- Add a 5% solution of alkaline potassium permanganate drop by drop to this solution.
- Does the colour of potassium permanganate persist when it is added initially?
- Why does the colour of potassium permanganate not disappear when excess is added?
Ans: Observations:
- Initial Addition: When a few drops of 5% alkaline potassium permanganate (KMnO₄, purple) are added to warm ethanol, the purple colour disappears. This indicates that KMnO₄ is reduced as it oxidizes ethanol to ethanoic acid (CH₃COOH).
- Excess Addition: When excess KMnO₄ is added, the purple colour persists because all the ethanol has been oxidized, and no more ethanol is available to react with the KMnO₄.
Explanation: Alkaline KMnO₄ is a strong oxidizing agent that adds oxygen to ethanol, converting it to ethanoic acid.
- Reaction: CH₃CH₂OH → CH₃COOH (in the presence of alkaline KMnO₄ + heat).
- Initially, KMnO₄ oxidizes ethanol, reducing itself (losing its purple colour). Once all ethanol is consumed, excess KMnO₄ remains unreacted, retaining its purple colour.
Conclusion: Ethanol is oxidized to ethanoic acid by alkaline KMnO₄, and the persistence of the purple colour indicates complete oxidation.
Activity 4.6: Reaction of Ethanol with Sodium
- Teacher’s demonstration –
- Drop a small piece of sodium, about the size of a couple of grains of rice, into ethanol (absolute alcohol).
- What do you observe? How will you test the gas evolved?
Ans: Observations:
- When a small piece of sodium is added to ethanol, it reacts, producing bubbles of a gas. The sodium dissolves, forming a solution.
- The gas evolved is hydrogen (H₂).
Test for Gas:
- Collect the gas by holding an inverted test tube over the reaction.
- Bring a lit splint near the mouth of the test tube. The gas burns with a “pop” sound, confirming it is hydrogen.
Explanation:
- Ethanol (CH₃CH₂OH) reacts with sodium (Na) to produce sodium ethoxide (CH₃CH₂ONa) and hydrogen gas.
- Reaction: 2Na(s) + 2CH₃CH₂OH(l) → 2CH₃CH₂ONa(aq) + H₂(g)
- The reaction is similar to that of metals with water, where hydrogen is evolved.
Conclusion: Sodium reacts with ethanol to produce hydrogen gas, which can be confirmed by the pop test, and sodium ethoxide.
Activity 4.7: Comparing Acidity of Ethanoic Acid and Hydrochloric Acid
- Compare the pH of dilute acetic acid and dilute hydrochloric acid using both litmus paper and universal indicator.
- Are both acids indicated by the litmus test?
- Does the universal indicator show them as equally strong acids?
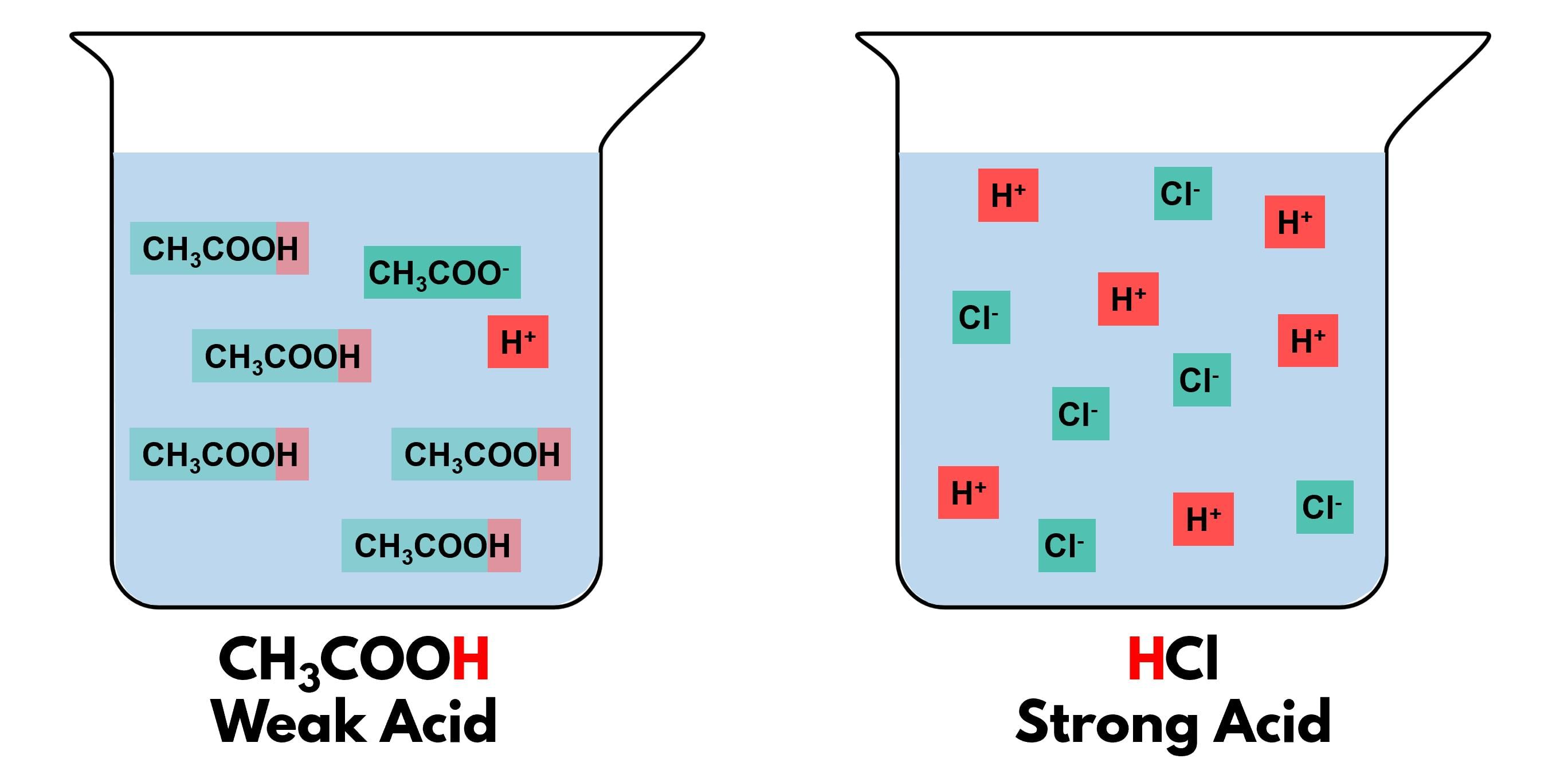
Ans: Observations:
Litmus Test:
- Dilute Acetic Acid (CH₃COOH): Red litmus remains red; blue litmus turns red.
- Dilute Hydrochloric Acid (HCl): Red litmus remains red; blue litmus turns red.
- Conclusion: Both acids turn blue litmus red, indicating they are acidic.
Universal Indicator Test:
- Dilute Acetic Acid: Shows a pH of ~3–4 (orange/yellow colour), indicating a weakly acidic solution.
- Dilute Hydrochloric Acid: Shows a pH of ~1–2 (red colour), indicating a strongly acidic solution.
- Conclusion: The universal indicator shows that HCl is a stronger acid (lower pH) than acetic acid.
Explanation:
- Both acetic acid and HCl are acids, as confirmed by the litmus test (blue to red).
- Acetic acid is a weak acid, partially ionizing in water (CH₃COOH ⇌ CH₃COO⁻ + H⁺), resulting in a higher pH.
- HCl is a strong acid, fully ionizing (HCl → H⁺ + Cl⁻), resulting in a lower pH.
Conclusion: Both acids are detected by litmus, but the universal indicator reveals HCl is stronger than acetic acid.
Activity 4.8: Esterification Reaction
- Take 1 mL ethanol (absolute alcohol) and 1 mL glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube.
- Warm in a water-bath for at least five minutes as shown in Fig. 4.11.
 Figure 4.11 Formation of ester
Figure 4.11 Formation of ester - Pour into a beaker containing 20–50 mL of water and smell the resulting mixture.
Ans: Observations:
- After warming the mixture of ethanol, glacial acetic acid, and concentrated sulphuric acid, a sweet, fruity smell is produced.
- When poured into water, the smell persists, indicating the formation of an ester.
Explanation: Ethanol (C₂H₅OH) reacts with ethanoic acid (CH₃COOH) in the presence of concentrated H₂SO₄ (a catalyst and dehydrating agent) to form ethyl ethanoate (CH₃COOC₂H₅), an ester, and water.
Reaction: CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
The sweet smell is characteristic of esters, which are used in perfumes and flavouring agents.
Conclusion: The reaction produces ethyl ethanoate, confirmed by its sweet, fruity smell, demonstrating esterification.
Activity 4.9: Reaction of Ethanoic Acid with Carbonates and Hydrogencarbonates
- Set up the apparatus as shown in Chapter 2, Activity 2.5.
- Take a spatula full of sodium carbonate in a test tube and add 2 mL of dilute ethanoic acid.
- What do you observe?
- Pass the gas produced through freshly prepared lime-water. What do you observe?
- Can the gas produced by the reaction between ethanoic acid and sodium carbonate be identified by this test?
- Repeat this activity with sodium hydrogencarbonate instead of sodium carbonate.
Ans: Observations:
With Sodium Carbonate (Na₂CO₃):
- Adding dilute ethanoic acid to sodium carbonate produces effervescence (bubbles) due to gas evolution.
- The gas, when passed through lime water (Ca(OH)₂), turns it milky due to the formation of calcium carbonate (CaCO₃).
- Reaction: 2CH₃COOH + Na₂CO₃ → 2CH₃COONa + H₂O + CO₂
With Sodium Hydrogencarbonate (NaHCO₃):
- Adding dilute ethanoic acid to sodium hydrogencarbonate also produces effervescence.
- The gas turns lime water milky, confirming it is carbon dioxide (CO₂).
- Reaction: CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂
Lime Water Test:
- Reaction: Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l) (milky precipitate).
- The gas is identified as CO₂ by this test.
Conclusion: Ethanoic acid reacts with both sodium carbonate and hydrogencarbonate to produce CO₂, confirmed by the lime water test, along with sodium acetate and water.
Activity 4.10: Effect of Soap on Oil-Water Mixtures
- Take about 10 mL of water each in two test tubes. Add a drop of oil (cooking oil) to both the test tubes and label them as A and B.
- To test tube B, add a few drops of soap solution. Now shake both the test tubes vigorously for the same period of time.
- Can you see the oil and water layers separately in both the test tubes immediately after you stop shaking them?
- Leave the test tubes undisturbed for some time and observe. Does the oil layer separate out? In which test tube does this happen first?
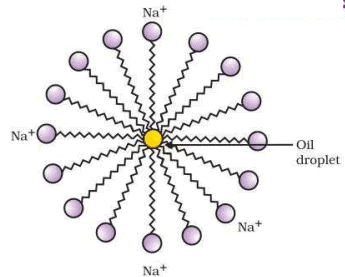 Figure 4.12 Formation of micelles
Figure 4.12 Formation of micelles
Ans: Observations:
Immediately After Shaking:
- Test Tube A (Water + Oil): Oil and water form an emulsion (cloudy mixture) but begin to separate quickly.
- Test Tube B (Water + Oil + Soap): A stable emulsion forms, and the mixture remains cloudy longer due to soap micelles.
After Standing:
- Test Tube A: Oil and water layers separate distinctly, with oil floating on top, within a short time.
- Test Tube B: The emulsion is more stable, and separation takes longer. The oil layer separates more slowly than in Test Tube A.
- Separation Speed: Separation happens first in Test Tube A.
Explanation:
- Oil and water are immiscible. Shaking creates a temporary emulsion, but they separate quickly in Test Tube A.
- In Test Tube B, soap molecules (sodium or potassium salts of long-chain carboxylic acids) form micelles. The hydrophobic tails of soap interact with oil, while the hydrophilic heads interact with water, stabilizing the emulsion.
- Micelle Formation: Soap micelles trap oil droplets, delaying separation.
Conclusion: Soap stabilizes oil-water emulsions by forming micelles, causing slower separation in Test Tube B compared to Test Tube A.
Activity 4.11: Comparing Soap Action in Hard and Soft Water
- Take about 10 mL of distilled water (or rainwater) and 10 mL of hard water (from a tubewell or hand-pump) in separate test tubes.
- Add a couple of drops of soap solution to both.
- Shake the test tubes vigorously for an equal period of time and observe the amount of foam formed.
- In which test tube do you get more foam? In which test tube do you observe a white curdy precipitate?
- Note for the teacher: If hard water is not available, prepare some hard water by dissolving hydrogencarbonates/ sulphates/ chlorides of calcium or magnesium in water.
Ans: Observations:
Distilled Water (Soft Water):
- Produces a large amount of foam after shaking with soap solution.
- No precipitate is observed.
Hard Water:
- Produces little or no foam after shaking.
- A white curdy precipitate (scum) is observed.
Explanation:
Soft Water: Contains no or minimal calcium (Ca²⁺) or magnesium (Mg²⁺) ions, allowing soap (sodium salts of long-chain carboxylic acids) to form micelles and produce foam easily.
Hard Water:Contains Ca²⁺ or Mg²⁺ ions, which react with soap to form insoluble calcium or magnesium salts (scum), reducing foam formation.
Reaction (Example): 2RCOONa + Ca²⁺ → (RCOO)₂Ca (insoluble scum) + 2Na⁺
The precipitate is the white curdy scum formed in hard water.
Conclusion: More foam is produced in distilled (soft) water. A white curdy precipitate forms in hard water due to soap reacting with Ca²⁺ or Mg²⁺ ions.
Activity 4.12: Comparing Soap and Detergent in Hard Water
- Take two test tubes with about 10 mL of hard water in each.
- Add five drops of soap solution to one and five drops of detergent solution to the other.
- Shake both test tubes for the same period.
- Do both test tubes have the same amount of foam?
- In which test tube is a curdy solid formed?
Ans: Observations:
Test Tube with Soap Solution:
- Produces little or no foam.
- A white curdy solid (scum) is formed.
Test Tube with Detergent Solution:
- Produces a significant amount of foam.
- No curdy solid is formed.
Explanation:
Soap in Hard Water: Soap (sodium salts of long-chain carboxylic acids) reacts with Ca²⁺ or Mg²⁺ ions in hard water, forming insoluble scum (e.g., (RCOO)₂Ca), which reduces foam.
Reaction: 2RCOONa + Ca²⁺ → (RCOO)₂Ca (scum) + 2Na⁺
Detergent in Hard Water: Detergents (e.g., sodium salts of sulphonic acids or ammonium salts) do not form insoluble precipitates with Ca²⁺ or Mg²⁺ ions, allowing them to produce foam effectively even in hard water.
Conclusion: The detergent test tube produces more foam and no curdy solid, while the soap test tube produces less foam and a curdy solid (scum).
|
80 videos|569 docs|80 tests
|
FAQs on NCERT Based Activity: Carbon and its Compounds - Science Class 10
| 1. What are carbon-based materials and why are they important? |  |
| 2. What is a homologous series of carbon compounds? |  |
| 3. How does ethanol react with sodium? |  |
| 4. How does the acidity of ethanoic acid compare to hydrochloric acid? |  |
| 5. What is the process of esterification, and why is it important? |  |
















