NCERT Based Activity : Chemical Reactions and Equations | Science Class 10 PDF Download
Activity 1.1 : Burning Magnesium Ribbon
CAUTION: This Activity needs the teacher’s assistance. It would be better if students wear suitable eyeglasses.
- Clean a magnesium ribbon about 3-4 cm long by rubbing it with sandpaper.
- Hold it with a pair of tongs. Burn it using a spirit lamp or burner and collect the ash so formed in a watch-glass as shown in Fig. . Burn the magnesium ribbon keeping it away as far as possible from your eyes.
- What do you observe?
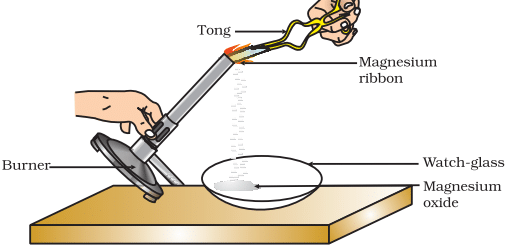 Burning of a magnesium ribbon in air and collection of magnesium oxide in a watch-glass
Burning of a magnesium ribbon in air and collection of magnesium oxide in a watch-glass
Observations:
- The magnesium ribbon burns with a bright, intense white light.
- A white ash is formed during the burning process which is magnesium oxide (MgO).
- The burning process releases a lot of heat and light, indicating a highly exothermic reaction.
- The magnesium ribbon may sparkle during the burning due to the high temperature.
Chemical Reaction occured is : 2Mg(s)+O2(g)→2MgO(s)
Magnesium + Oxygen → Magnesium oxide
(Reactants) (Product)
Explanation:
- Magnesium (Mg) reacts with oxygen (O₂) from the air.
- The reaction is highly exothermic, meaning it releases a large amount of heat and light.
- Magnesium oxide (MgO) is formed as a white solid ash.
This reaction demonstrates the process of oxidation, where magnesium undergoes a chemical change due to its reaction with oxygen in the air.
Activity 1.2: Reaction between Lead Nitrate and Potassium Iodide
- Take lead nitrate solution in a test tube.
- Add potassium iodide solution to this.
- What do you observe?
Observation: A yellow precipitate of lead iodide (PbI2) forms immediately.
Chemical Reaction: Pb(NO3)2 (aq)+2KI(aq)→PbI2 (s)+2KNO3(aq)
Explanation:
- Lead nitrate (Pb(NO3)2) reacts with potassium iodide (KI) to form lead iodide (PbI2) as a yellow precipitate and potassium nitrate (KNO₃) in solution.
- This is an example of a double displacement reaction or a precipitation reaction, where an insoluble product (PbI2) is formed when two aqueous solutions are mixed.
Activity 1.3: Reaction of Zinc with Dilute Hydrochloric Acid or Sulphuric Acid
- Take a few zinc granules in a conical flask or a test tube.
- Add dilute hydrochloric acid or sulphuric acid to this (Fig.). CAUTION: Handle the acid with care.
- Do you observe anything happening around the zinc granules?
- Touch the conical flask or test tube. Is there any change in its temperature?
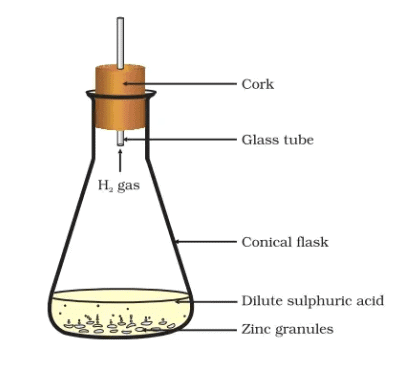 Formation of hydrogen gas by the action of dilute sulphuric acid on zinc
Formation of hydrogen gas by the action of dilute sulphuric acid on zinc
Observation:
- When the acid is added to the zinc granules, bubbles of gas are produced around the zinc.
- The gas is hydrogen gas (H₂), which is released as a result of the reaction between zinc and the acid.
The reaction is exothermic, meaning it releases heat. When you touch the conical flask or test tube, you will feel that it has become warm or hot.
Chemical Reaction:
Zn (s)+2HCl(aq)→ZnCl2 (aq)+H2(g)
or
Zn (s)+H2SO4(aq)→ZnSO4 (aq)+H2(g)
Explanation:
- Zinc reacts with the dilute acid (HCl or H2SO4) to form zinc chloride (ZnCl2) or zinc sulfate (ZnSO4) in solution, and hydrogen gas (H2) is produced.
- The production of hydrogen gas and the release of heat indicate that the reaction is exothermic.
This activity demonstrates an example of a single displacement reaction and helps to understand the concept of exothermic reactions.
Activity 1.4 : Reaction of Calcium Oxide (Quick Lime) with Water
- Take a small amount of calcium oxide or quick lime in a beaker.
- Slowly add water to this.
- Touch the beaker as shown in.
- Do you feel any change in temperature?
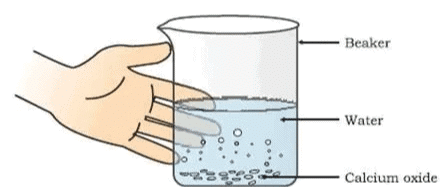 Formation of slaked lime by the reaction of calcium oxide with water
Formation of slaked lime by the reaction of calcium oxide with water
Observation:
When water is added to calcium oxide, you will observe that the beaker becomes very hot.
This indicates that the reaction is exothermic, meaning it releases heat.
Chemical Reaction: CaO (s)+H2O (l)→Ca(OH)2 (aq)
Explanation:
- Calcium oxide (quick lime) reacts with water to form calcium hydroxide (slaked lime), releasing a significant amount of heat during the process.
- The heat released is enough to make the beaker feel hot to the touch, demonstrating the exothermic nature of the reaction.
This reaction is an example of a combination reaction and shows how water and calcium oxide react to produce calcium hydroxide while releasing heat.
Activity 1.5: Heating of Ferrous Sulphate Crystals
- Take about 2 g ferrous sulphate crystals in a dry boiling tube.
- Note the colour of the ferrous sulphate crystals.
- Heat the boiling tube over the flame of a burner or spirit lamp as shown in Fig.
- Observe the colour of the crystals after heating.
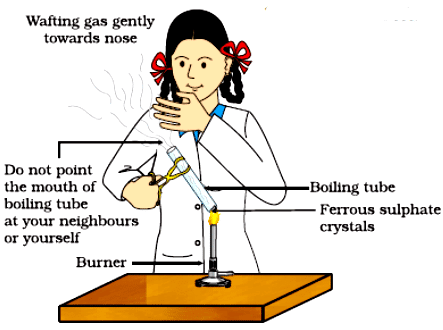 Correct way of heating the boiling tube containing crystals of ferrous sulphate and of smelling the odour
Correct way of heating the boiling tube containing crystals of ferrous sulphate and of smelling the odour
Observation:
- Initially, the ferrous sulphate crystals are green.
- After heating, the colour changes to brown.
- This change is due to the formation of ferric oxide (Fe₂O₃) and the release of sulfur dioxide (SO₂) and sulfur trioxide (SO₃) gases.
Chemical Reaction: 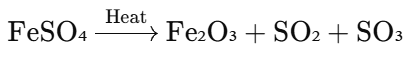
Explanation:
- When ferrous sulphate is heated, it undergoes decomposition to form ferric oxide (Fe₂O₃), sulfur dioxide (SO₂), and sulfur trioxide (SO₃).
- The green color of ferrous sulphate (FeSO₄) is due to the presence of Fe²⁺ ions, and upon heating, it is oxidized to form ferric oxide (Fe₂O₃), which is brown.
- This is an example of a thermal decomposition reaction.
This activity demonstrates the decomposition of ferrous sulphate when heated and the change in color as a result of the chemical reaction.
Activity 1.6 : Heating of Lead Nitrate
- Take about 2 g lead nitrate powder in a boiling tube.
- Hold the boiling tube with a pair of tongs and heat it over a flame, as shown in Fig.
- What do you observe? Note down the change, if any.
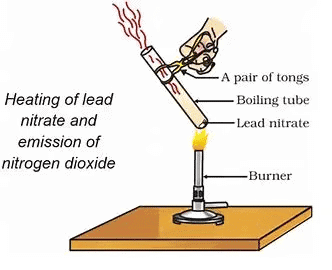 Heating of lead nitrate and emission of nitrogen dioxideObservation:
Heating of lead nitrate and emission of nitrogen dioxideObservation:
- Upon heating, lead nitrate undergoes decomposition and decomposes into lead monoxide (PbO), nitrogen dioxide (NO₂), and oxygen gas (O₂).
- A yellow color appears in the boiling tube due to the formation of lead monoxide (PbO).
- You will also observe brown fumes of nitrogen dioxide (NO₂) being released.
- Oxygen gas is released during the reaction.
Chemical Reaction: 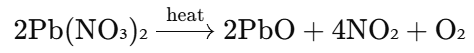
Explanation:
- Lead nitrate decomposes when heated to form lead monoxide (PbO), nitrogen dioxide (NO₂), and oxygen (O₂).
- The brown fumes are due to the formation of nitrogen dioxide, a toxic gas, which also gives a characteristic color.
- This is an example of a decomposition reaction where a single reactant (lead nitrate) breaks down into multiple products.
Safety Note: Ensure proper ventilation while performing this activity as nitrogen dioxide is toxic and can irritate the respiratory system.
Activity 1.7: Electrolysis of Water
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in Fig.
- Connect these electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
- Is the volume of the gas collected the same in both the test tubes?
- Once the test tubes are filled with the respective gases, remove them carefully.
- Test these gases one by one by bringing a burning candle close to the mouth of the test tubes. CAUTION: This step must be performed carefully by the teacher.
- What happens in each case?
- Which gas is present in each test tube?
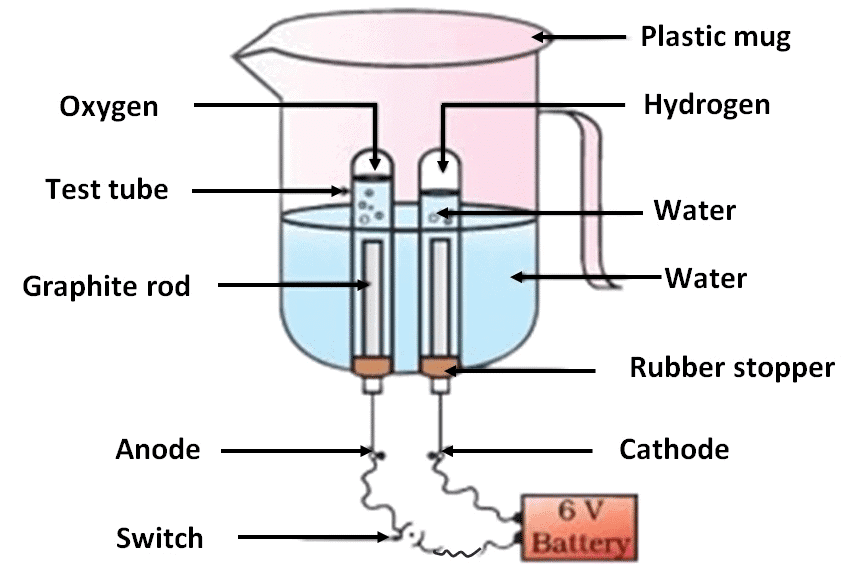
Observation:
- You will observe bubbles forming at both electrodes.
- The gas collected at one electrode will extinguish the candle, while the gas collected at the other will cause the candle to burn more brightly.
- The volume of gas collected at the two electrodes will not be the same. The volume of gas collected at the anode will be half of that collected at the cathode.
Gas Testing:
- Gas at the cathode: When a burning candle is brought near the test tube, it will burn more brightly, indicating that the gas is hydrogen (H₂).
- Gas at the anode: When a burning candle is brought near the test tube, it will be extinguished, indicating that the gas is oxygen (O₂).
Chemical Reaction: 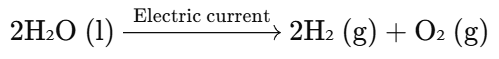
Explanation:
- This is an example of electrolysis of water, where water is split into hydrogen and oxygen gases due to the application of electric current.
- Hydrogen gas is produced at the cathode (negative electrode), and oxygen gas is produced at the anode (positive electrode).
Activity 1.8: Decomposition of Silver Chloride in Sunlight
- Take about 2 g silver chloride in a china dish.
- What is its colour?
- Place this china dish in sunlight for some time (Fig. ).
- Observe the colour of the silver chloride after some time.
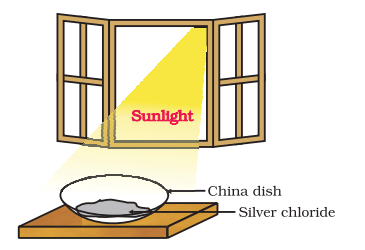 Silver chloride turns grey in sunlight to form silver metal
Silver chloride turns grey in sunlight to form silver metal
Observation:
- Initially, the silver chloride is white.
- After exposing it to sunlight, the silver chloride turns grey.
- This color change occurs due to the decomposition of silver chloride into silver metal (Ag) and chlorine gas (Cl₂) when exposed to sunlight.
Chemical Reaction: 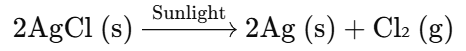
Explanation:
- The decomposition reaction is driven by the energy from sunlight, which breaks down silver chloride into silver and chlorine gas.
- This is a classic example of a photochemical reaction.
Observation:
- When you touch the bottom of the test tube, you will feel that it is cold.
- This indicates that the reaction is absorbing heat from the surroundings.
Conclusion: The reaction between barium hydroxide and ammonium chloride is endothermic, as it absorbs heat from the surroundings, making the test tube feel cold.
Chemical Reaction: Ba(OH)₂ (s)+2NH₄Cl (s)→BaCl₂ (aq)+2NH₃ (g)+2H₂O (l)
Explanation: In this reaction, barium hydroxide reacts with ammonium chloride to form barium chloride, ammonia gas, and water. The absorption of heat during the reaction makes it an endothermic process.
Activity 1.9: Displacement Reaction of Iron Nails in Copper Sulphate Solution
- Take three iron nails and clean them by rubbing with sand paper.
- Take two test tubes marked as (A) and (B). In each test tube, take about 10 mL copper sulphate solution.
- Tie two iron nails with a thread and immerse them carefully in the copper sulphate solution in test tube B for about 20 minutes [Fig.(a)]. Keep one iron nail aside for comparison.
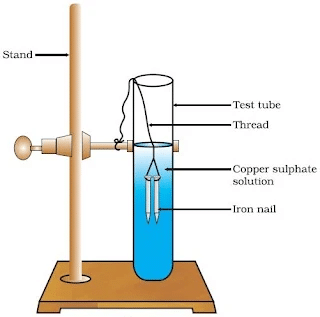 (a) Iron nails dipped in copper sulphate solution
(a) Iron nails dipped in copper sulphate solution
- After 20 minutes, take out the iron nails from the copper sulphate solution.
- Compare the intensity of the blue colour of copper sulphate solutions in test tubes (A) and (B) [Fig.(b)].
- Also, compare the colour of the iron nails dipped in the copper sulphate solution with the one kept aside [Fig.(b)]
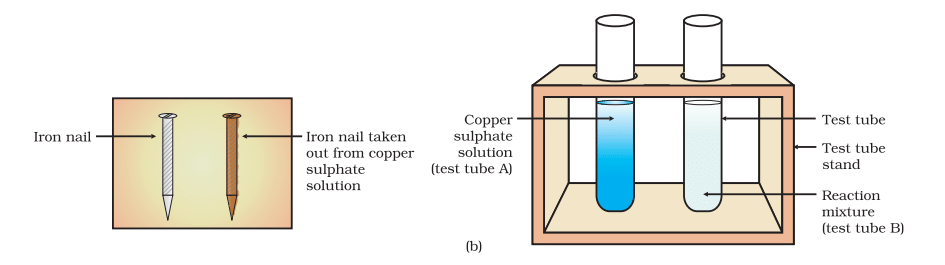 (b) Iron nails and copper sulphate solutions compared before and after the experiment
(b) Iron nails and copper sulphate solutions compared before and after the experiment
- The copper sulfate solution in test tube A will maintain its blue color, while the solution in test tube B will show a decrease in blue intensity, as the copper ions (Cu²⁺) from the copper sulfate solution are displaced by iron ions (Fe²⁺) and copper metal is deposited on the iron nails.
- The iron nails in test tube B will appear to have a reddish-brown color due to the deposition of copper metal on the surface of the nails.
- The iron nail kept aside (in test tube A) will remain its original metallic color (grayish-silver).
Chemical Reaction: Fe (s)+CuSO₄ (aq)→FeSO₄ (aq)+Cu (s)
Explanation:
- This is an example of a displacement reaction. Iron (Fe) displaces copper (Cu) from copper sulfate (CuSO₄) because iron is more reactive than copper.
- During the reaction, copper ions (Cu²⁺) from the solution are reduced to copper metal (Cu), which gets deposited on the iron nails, while iron is oxidized to form iron sulfate (FeSO₄) in solution.
- The decrease in the blue color intensity of the copper sulfate solution indicates the displacement of copper ions by iron ions.
Activity 1.10 : Reaction between Sodium Sulphate and Barium Chloride
- Take about 3 mL of sodium sulphate solution in a test tube.
- In another test tube, take about 3 mL of barium chloride solution.
- Mix the two solutions (Fig.).
- What do you observe?
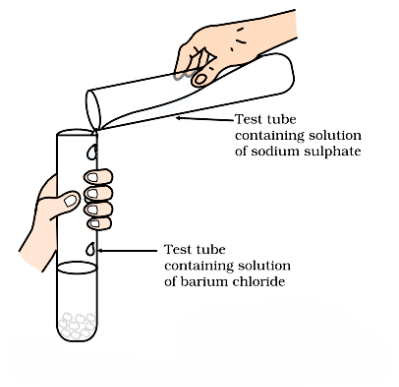 Formation of barium sulphate and sodium chloride
Formation of barium sulphate and sodium chloride
Observation:
- A white precipitate forms immediately when the two solutions are mixed.
- The precipitate is barium sulfate (BaSO₄), which is insoluble in water.
Chemical Reaction: Na₂SO₄ (aq)+BaCl₂ (aq)→BaSO₄ (s)+2NaCl (aq)
Explanation:
- This is an example of a precipitation reaction, where barium sulfate (BaSO₄) is formed as a solid precipitate due to the reaction between sodium sulfate (Na₂SO₄) and barium chloride (BaCl₂).
- The white color of the precipitate indicates the formation of barium sulfate, which is insoluble in water and precipitates out of the solution.
Recall Activity 1.2, where you have mixed the solutions of lead(II) nitrate and potassium iodide.
(i) What was the colour of the precipitate formed? Can you name the compound precipitated?
(ii) Write the balanced chemical equation for this reaction.
(iii) Is this also a double displacement reaction?
Ans:
(i) A yellow precipitate of lead iodide (PbI2) forms immediately.
(ii) Chemical Reaction: Pb(NO3)2 (aq)+2KI(aq)→PbI2 (s)+2KNO3(aq)
(iii) Explanation:
- Lead nitrate (Pb(NO3)2) reacts with potassium iodide (KI) to form lead iodide (PbI2) as a yellow precipitate and potassium nitrate (KNO₃) in solution.
- This is an example of a double displacement reaction or a precipitation reaction, where an insoluble product (PbI2) is formed when two aqueous solutions are mixed.
Activity 1.11: Heating of Copper Powder
- Heat a china dish containing about 1 g copper powder (Fig.).
- What do you observe?
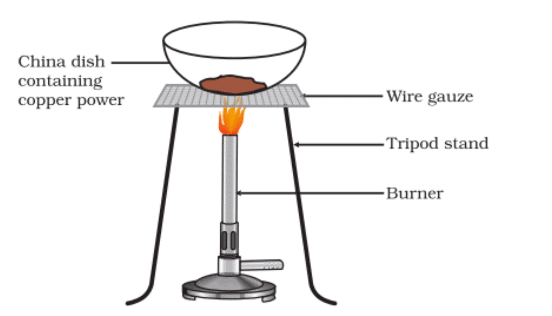 Oxidation of copper to copper oxide
Oxidation of copper to copper oxide
Observation:
- Upon heating, the copper powder does not undergo any significant change in appearance.
- Copper powder will remain its original reddish-brown color even after heating.
- No visible reaction or color change is observed because copper does not react with oxygen at low temperatures.
Explanation:
- Copper metal is generally unreactive with air at room temperature or under mild heating. However, if heated to a very high temperature, copper can react with oxygen to form copper oxide (CuO), which is black.
- Since copper is not heated to a high enough temperature in this activity, no change occurs during the heating process.
|
80 videos|569 docs|80 tests
|
FAQs on NCERT Based Activity : Chemical Reactions and Equations - Science Class 10
| 1. What is the observation when magnesium ribbon is burned? |  |
| 2. What happens when lead nitrate reacts with potassium iodide? |  |
| 3. What is the result of the reaction between zinc and dilute hydrochloric acid? |  |
| 4. What is formed when calcium oxide reacts with water? |  |
| 5. What is the significance of heating lead nitrate? |  |

















