NCERT Based Activity: Metal and Non-metals | Science Class 10 PDF Download
Activity 3.1: Observing Metallic Lustre
- Take samples of iron, copper, aluminium and magnesium. Note the appearance of each sample.
- Clean the surface of each sample by rubbing them with sand paper and note their appearance again.
Ans: Initial Appearance: The samples may appear dull or tarnished due to surface oxidation or impurities.
After Cleaning: After rubbing with sandpaper, the samples will exhibit a shiny, metallic surface. This is because metals in their pure state have a property called metallic lustre, which is revealed once the oxide layer or impurities are removed. Observation for Each Metal:
Observation for Each Metal:
- Iron: Dull grey initially, shiny silver-grey after cleaning.
- Copper: Brownish or greenish (due to patina) initially, shiny reddish-brown after cleaning.
- Aluminium: Dull silver initially, bright silver after cleaning.
- Magnesium: Dull silver initially, shiny silver after cleaning.
Activity 3.2: Testing Hardness of Metals
- Take small pieces of iron, copper, aluminium, and magnesium. Try to cut these metals with a sharp knife and note your observations.
- Hold a piece of sodium metal with a pair of tongs.
- CAUTION: Always handle sodium metal with care. Dry it by pressing between the folds of a filter paper.
- Put it on a watch-glass and try to cut it with a knife.
- What do you observe?
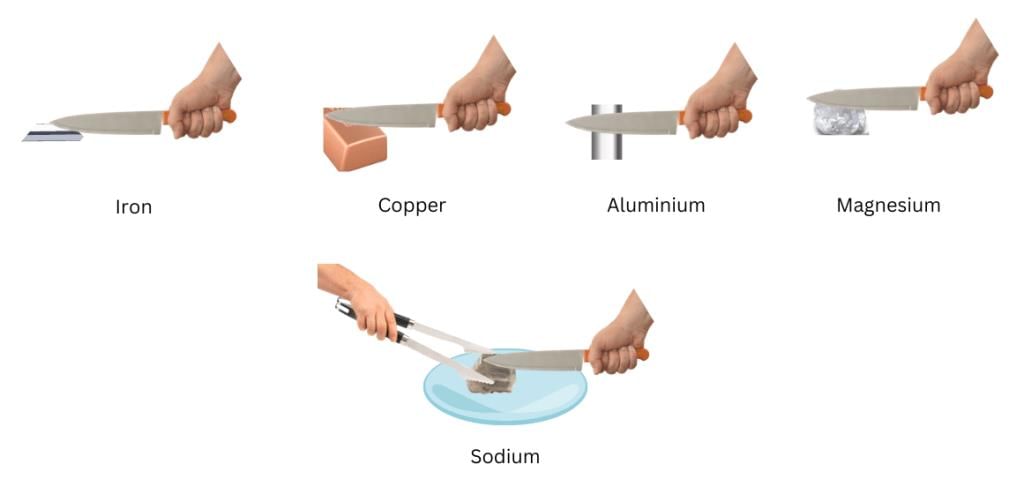
Ans: Iron, Copper, Aluminium, Magnesium:
- These metals are generally hard and cannot be easily cut with a sharp knife. The knife may leave scratches or slight marks, but significant cutting is difficult.
- Iron: Very hard, resists cutting.
- Copper: Moderately hard, slightly easier to scratch than iron but still resists cutting.
- Aluminium: Softer than iron and copper, may show slight indentation but not easily cut.
- Magnesium: Relatively soft compared to iron but still requires effort to cut; may show some deformation.
Sodium: - Sodium is very soft and can be easily cut with a knife. When cut, it reveals a shiny, silvery surface that quickly tarnishes due to reaction with air (forming sodium oxide or hydroxide).
- Observation: The knife cuts through sodium like butter, and the freshly cut surface is shiny but dulls rapidly due to oxidation.
Conclusion: Metals vary in hardness. Sodium (an alkali metal) is exceptionally soft, while others like iron are much harder.
Activity 3.3: Testing Malleability of Metals
- Take pieces of iron, zinc, lead and copper.
- Place any one metal on a block of iron and strike it four or five times with a hammer. What do you observe?
- Repeat with other metals.
- Record the change in the shape of these metals.
 Metals
Metals
Ans: Observations:
- Iron: When struck, iron deforms slightly but does not flatten easily due to its hardness. It may show dents or slight bending.
- Zinc: Zinc is softer and more malleable than iron. It flattens or forms thin sheets when struck repeatedly.
- Lead: Lead is very soft and highly malleable. It flattens significantly into thin sheets with minimal effort.
- Copper: Copper is malleable and flattens into thin sheets when struck, though it requires more force than lead.
Conclusion: The ability of metals to be beaten into thin sheets is called malleability. Lead and copper are highly malleable, zinc is moderately malleable, and iron is less malleable due to its hardness. The text notes that gold and silver are the most malleable metals, though they are not tested here.
Activity 3.4: Observing Ductility in Daily Life
List the metals whose wires you have seen in daily life.
Ans: Common Metals Used for Wires:
- Copper: Widely used in electrical wiring due to its excellent conductivity and ductility.
- Aluminium: Used in overhead power lines because it is lightweight, ductile, and a good conductor.
- Gold: Used in high-precision electronics (e.g., connectors) due to its extreme ductility and resistance to corrosion (though less common in daily life).
- Silver: Occasionally used in specialized applications due to its high conductivity, though rare due to cost.
Explanation: The ability of metals to be drawn into thin wires is called ductility. Copper and aluminium are the most commonly observed in household and industrial wiring. Gold is noted as the most ductile metal, capable of being drawn into a 2 km wire from just 1 gram.
Activity 3.5: Observing Ductility in Daily Life
- Take an aluminium or copper wire. Clamp this wire on a stand, as shown in Fig. 3.1.
- Fix a pin to the free end of the wire using wax. n Heat the wire with a spirit lamp, candle or a burner near the place where it is clamped.
- What do you observe after some time?
- Note your observations. Does the metal wire melt?
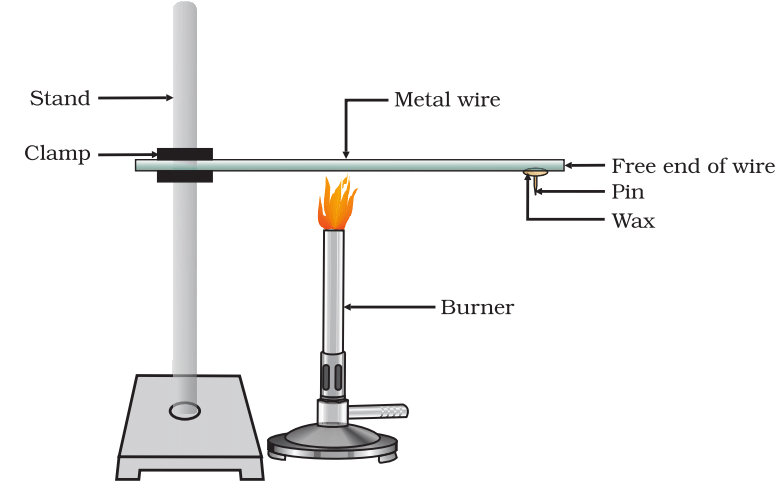 Figure 3.1 Metals are good conductors of heat.
Figure 3.1 Metals are good conductors of heat.
Observation:
- After some time, the pin falls off.
- The metal wire does not melt.
Explanation:
- Metals are good conductors of heat.
- Heat travels from the heated end to the free end of the wire.
- The wax melts due to the transferred heat, causing the pin to fall.
- The metal wire does not melt because: Copper melts at approximately 1085°C. Aluminium melts at approximately 660°C.
- These melting points are much higher than the temperature produced by a candle or spirit lamp (typically less than 1000°C).
Conclusion: Heat travels through metals by conduction. Aluminium and copper are excellent conductors of heat. This experiment demonstrates heat conduction in metals and shows that they do not melt easily under ordinary heating conditions.
Activity 3.6: Testing Electrical Conductivity of Metals
- Set up an electric circuit as shown in Fig. 3.2.
- Place the metal to be tested in the circuit between terminals A and B as shown.
- Does the bulb glow? What does this indicate?
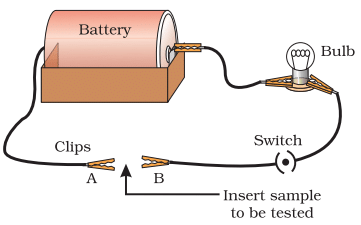 Metals are good conductors of electricity.
Metals are good conductors of electricity.
Ans: Setup: The circuit includes a battery, a bulb, wires, and terminals A and B where the metal sample is inserted.
Observations:
- When metals like iron, copper, aluminium, magnesium, zinc, or lead are placed between terminals A and B, the bulb glows.
- This indicates that these metals are good conductors of electricity, allowing current to flow through the circuit and light the bulb.
Explanation: Metals conduct electricity due to the presence of free electrons in their structure. The glowing bulb confirms this property.
Additional Note: The text mentions that electric wires are coated with PVC or rubber-like materials to insulate them, preventing unwanted current flow or short circuits.
Activity 3.7: Comparing Physical Properties of Non-Metals
- Collect samples of carbon (coal or graphite), sulphur and iodine. Carry out the Activities 3.1 to 3.4 and 3.6 with these non-metals and record your observations.
Ans: Activity 3.1 (Appearance and Lustre):
- Carbon (Coal): Dull, black, non-shiny surface. No change after rubbing with sandpaper.
- Carbon (Graphite): Dark grey, slightly shiny (lustrous) surface, retains lustre after rubbing.
- Sulphur: Dull yellow, non-shiny surface, no lustre even after rubbing.
- Iodine: Shiny, blackish-purple crystals, exhibits lustre (an exception for non-metals).
Activity 3.2 (Hardness): - Carbon (Coal): Brittle, breaks easily when struck, cannot be cut with a knife.
- Carbon (Graphite): Soft, leaves marks when scratched (used in pencils), can be cut but crumbles.
- Sulphur: Brittle, breaks into powder when struck or cut.
- Iodine: Brittle, breaks into small pieces when struck or cut.
Activity 3.3 (Malleability):
- None of these non-metals (carbon, sulphur, iodine) can be beaten into thin sheets. They are brittle and shatter or crumble when struck with a hammer.
Activity 3.4 (Ductility):
- Non-metals cannot be drawn into wires. Carbon, sulphur, and iodine lack ductility and break when stretched or pulled.
Activity 3.6 (Conductivity):
- Carbon (Coal): Does not conduct electricity; the bulb does not glow.
- Carbon (Graphite): Conducts electricity (an exception for non-metals); the bulb glows.
- Sulphur: Does not conduct electricity; the bulb does not glow.
- Iodine: Does not conduct electricity; the bulb does not glow.
Table 3.1 Compilation: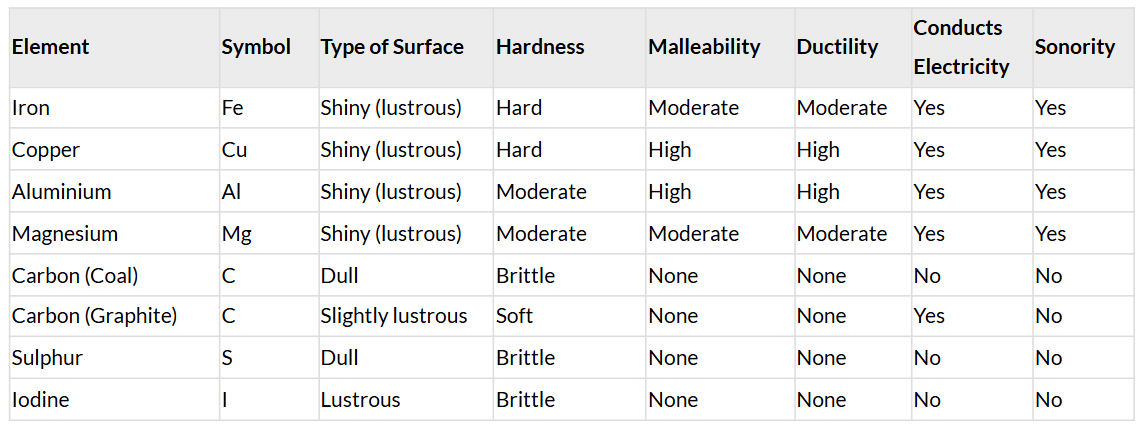
Activity 3.8: Testing Acidity/Basicity of Metal and Non-Metal Oxides
- Take a magnesium ribbon and some sulphur powder.
- Burn the magnesium ribbon. Collect the ashes formed and dissolve them in water.
- Test the resultant solution with both red and blue litmus paper.
- Is the product formed on burning magnesium acidic or basic?
- Now burn sulphur powder. Place a test tube over the burning sulphur to collect the fumes produced.
- Add some water to the above test tube and shake.
- Test this solution with blue and red litmus paper.
- Is the product formed on burning sulphur acidic or basic?
- Can you write equations for these reactions?
Ans:
1. Magnesium:
Burning: Magnesium ribbon burns with a dazzling white flame, producing white ash (magnesium oxide, MgO).
Dissolving in Water: Magnesium oxide reacts with water to form magnesium hydroxide (Mg(OH)₂), a weak base.
Reaction: MgO(s) + H₂O(l) → Mg(OH)₂(aq)
Litmus Test: Red Litmus: Turns blue, indicating a basic solution. Blue Litmus: Remains blue (no change).
Conclusion: The product (magnesium hydroxide) is basic.
Equations:
- Burning: 2Mg(s) + O₂(g) → 2MgO(s)
- Dissolving: MgO(s) + H₂O(l) → Mg(OH)₂(aq)
2. Sulphur:
Burning: Sulphur burns with a blue flame, producing sulphur dioxide (SO₂) gas, which is collected in the test tube.
Dissolving in Water: Sulphur dioxide reacts with water to form sulphurous acid (H₂SO₃), a weak acid.
Reaction: SO₂(g) + H₂O(l) → H₂SO₃(aq)
Litmus Test: Blue Litmus: Turns red, indicating an acidic solution. Red Litmus: Remains red (no change).
Conclusion: The product (sulphurous acid) is acidic.
Equations:
- Burning: S(s) + O₂(g) → SO₂(g)
- Dissolving: SO₂(g) + H₂O(l) → H₂SO₃(aq)
General Conclusion: Most metals form basic oxides (e.g., MgO), while most non-metals form acidic oxides (e.g., SO₂).
Activity 3.9: Burning Metals in Air
- CAUTION: The following activity needs the teacher’s assistance. It would be better if students wear eye protection.
- Hold any of the samples taken above with a pair of tongs and try burning over a flame. Repeat with the other metal samples.
- Collect the product if formed.
- Let the products and the metal surface cool down.
- Which metals burn easily?
- What flame colour did you observe when the metal burnt?
- How does the metal surface appear after burning?
- Arrange the metals in the decreasing order of their reactivity towards oxygen.
- Are the products soluble in water?
Ans: Metals Tested: Aluminium, copper, iron, lead, magnesium, zinc, sodium.
Observations:
- Sodium: Burns vigorously with a yellow flame, producing white sodium oxide (Na₂O). The surface may appear oxidized. Highly reactive.
- Magnesium: Burns with a dazzling white flame, producing white magnesium oxide (MgO). The surface is coated with white ash. Very reactive.
- Iron: Does not burn as a solid but iron filings burn vigorously with a sparkling effect, forming iron oxides (e.g., Fe₃O₄). The surface may appear blackened.
- Zinc: Burns with a bluish-green flame, forming zinc oxide (ZnO). user: The surface is coated with white ash.
- Aluminium: Burns with a white flame when powdered, forming aluminium oxide (Al₂O₃). Solid aluminium may only form a thin oxide layer unless heated strongly.
- Lead: Does not burn easily but may form a thin layer of lead oxide (PbO) on heating, appearing yellowish or grey.
- Copper: Does not burn but forms a black layer of copper(II) oxide (CuO) on the surface when heated.
Flame Colours:
- Sodium: Yellow
- Magnesium: White
- Zinc: Bluish-green
- Aluminium: White (when powdered)
- Iron: Sparkling (filings)
- Copper, Lead: No distinct flame (do not burn easily).
Surface After Burning:
- Sodium, Magnesium, Zinc: White ash or coating.
- Iron: Blackened or reddish-brown oxide layer.
- Aluminium: Thin white oxide layer.
- Copper: Black CuO layer.
- Lead: Yellowish or grey oxide layer.
Reactivity Order (Decreasing): Sodium > Magnesium > Zinc > Iron > Aluminium > Lead > Copper
(Sodium and magnesium burn easily; others react less vigorously or only form oxide layers.)
Solubility of Products:
- Sodium Oxide (Na₂O): Soluble, forms sodium hydroxide (NaOH).
Na₂O(s) + H₂O(l) → 2NaOH(aq) - Magnesium Oxide (MgO): Slightly soluble, forms magnesium hydroxide (Mg(OH)₂).
MgO(s) + H₂O(l) → Mg(OH)₂(aq) - Zinc Oxide (ZnO): Insoluble in water.
- Iron Oxides (Fe₃O₄, Fe₂O₃): Insoluble in water.
- Aluminium Oxide (Al₂O₃): Insoluble in water.
- Lead Oxide (PbO): Insoluble in water.
- Copper(II) Oxide (CuO): Insoluble in water.
Conclusion: Sodium and magnesium are the most reactive, burning easily. Most metal oxides are insoluble, except sodium oxide and, to a lesser extent, magnesium oxide.
Activity 3.10: Reactivity of Metals with Water
- CAUTION: This Activity needs the teacher’s assistance.
- Collect the samples of the same metals as in Activity 3.9.
- Put small pieces of the samples separately in beakers half-filled with cold water.
- Which metals reacted with cold water? Arrange them in the increasing order of their reactivity with cold water.
- Did any metal produce fire on water?
- Does any metal start floating after some time?
- Put the metals that did not react with cold water in beakers half-filled with hot water.
- For the metals that did not react with hot water, arrange the apparatus as shown in Fig. 3.3 and observe their reaction with steam.
- Which metals did not react even with steam? Arrange the metals in the decreasing order of reactivity with water.
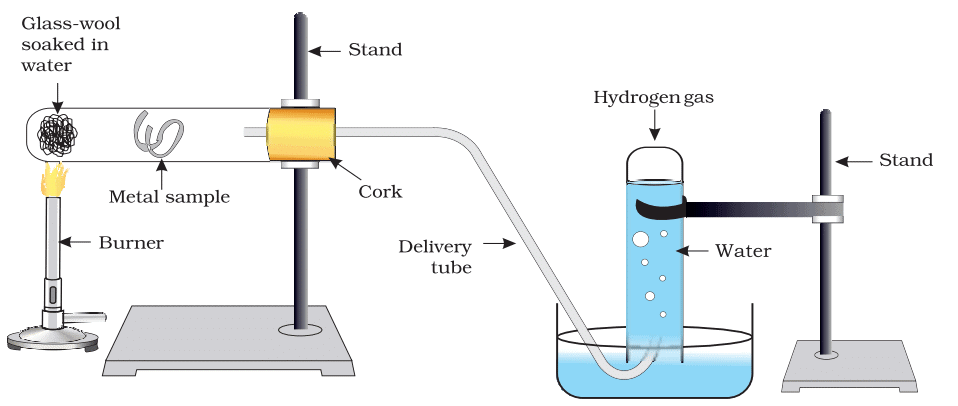 Figure 3.3 Action of steam on a metal
Figure 3.3 Action of steam on a metal
Ans: Metals Tested: Aluminium, copper, iron, lead, magnesium, zinc, sodium.
Cold Water:
- Sodium: Reacts violently, producing sodium hydroxide and hydrogen gas. The reaction is exothermic, and the hydrogen may catch fire.
2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g) + heat - Calcium: Reacts less violently, producing calcium hydroxide and hydrogen. Calcium floats due to hydrogen bubbles sticking to its surface.
Ca(s) + 2H₂O(l) → Ca(OH)₂(aq) + H₂(g) - Magnesium, Aluminium, Zinc, Iron, Lead, Copper: No reaction with cold water.
Reactivity with Cold Water (Increasing): Calcium < Sodium.
Fire on Water: Sodium may produce fire due to the ignition of hydrogen gas.
Floating: Calcium floats due to hydrogen bubbles. Sodium may also appear to move rapidly on the surface due to gas evolution.
Hot Water:
- Magnesium: Reacts slowly with hot water, forming magnesium hydroxide and hydrogen. It may float due to hydrogen bubbles.
Mg(s) + 2H₂O(l) → Mg(OH)₂(aq) + H₂(g) - Aluminium, Zinc, Iron, Lead, Copper: No reaction with hot water.
Steam:
- Magnesium: Reacts with steam to form magnesium oxide and hydrogen.
Mg(s) + H₂O(g) → MgO(s) + H₂(g) - Aluminium: Reacts with steam to form aluminium oxide and hydrogen.
2Al(s) + 3H₂O(g) → Al₂O₃(s) + 3H₂(g) - Zinc: Reacts with steam to form zinc oxide and hydrogen.
Zn(s) + H₂O(g) → ZnO(s) + H₂(g) - Iron: Reacts with steam to form iron oxide (Fe₃O₄) and hydrogen.
3Fe(s) + 4H₂O(g) → Fe₃O₄(s) + 4H₂(g) - Lead, Copper: No reaction with steam.
Metals with No Reaction (Even with Steam): Lead, Copper.
Reactivity Order (Decreasing): Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead = Copper (no reaction).
Conclusion: Sodium and calcium react with cold water, magnesium with hot water, and aluminium, zinc, and iron with steam. Lead and copper are unreactive with water in any form.
Activity 3.11: Reactivity of Metals with Dilute Hydrochloric Acid
- Collect all the metal samples except sodium and potassium again. If the samples are tarnished, rub them clean with sand paper.
- CAUTION: Do not take sodium and potassium as they react vigorously even with cold water.
- Put the samples separately in test tubes containing dilute hydrochloric acid.
- Suspend thermometers in the test tubes, so that their bulbs are dipped in the acid.
- Observe the rate of formation of bubbles carefully.
- Which metals reacted vigorously with dilute hydrochloric acid?
- With which metal did you record the highest temperature?
- Arrange the metals in the decreasing order of reactivity with dilute acids.
- Write equations for the reactions of magnesium, aluminium, zinc and iron with dilute hydrochloric acid.
Ans: Metals Tested: Aluminium, copper, iron, lead, magnesium, zinc.
Observations:
- Magnesium: Reacts vigorously, producing rapid bubbles of hydrogen gas and a significant temperature increase (most exothermic).
Mg(s) + 2HCl(aq) → Mg: Cl₂(aq) + H₂(g) - Aluminium: Reacts moderately after an initial delay (due to protective oxide layer), producing hydrogen bubbles and a temperature rise.
2Al(s) + 6HCl(aq) → 2AlCl₃(aq) + 3H₂(g) - Zinc: Reacts steadily, producing hydrogen bubbles and a moderate temperature increase.
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) - Iron: Reacts slowly, producing fewer bubbles and a slight temperature increase.
Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g) - Lead: Very slow or no reaction (due to low reactivity and formation of insoluble PbCl₂).
- Copper: No reaction; no bubbles or temperature change (copper is below hydrogen in the reactivity series).
Vigorous Reaction: Magnesium reacts most vigorously.
Highest Temperature: Magnesium, due to the fastest and most exothermic reaction.
Reactivity Order (Decreasing): Magnesium > Aluminium > Zinc > Iron > Lead > Copper.
Equations:
- Magnesium: Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
- Aluminium: 2Al(s) + 6HCl(aq) → 2AlCl₃(aq) + 3H₂(g)
- Zinc: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
- Iron: Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g)
Note: Copper does not react with dilute HCl. Lead’s reaction is negligible due to the formation of insoluble lead chloride.
Activity 3.12: Displacement Reactions with Metal Salts
- Take a clean wire of copper and an iron nail.
- Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in test tubes (Fig. 3.4).
- Record your observations after 20 minutes.
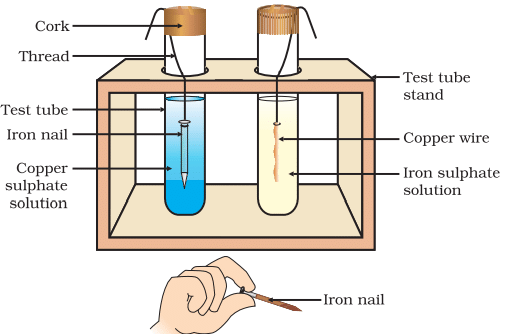
- In which test tube did you find that a reaction has occurred?
- On what basis can you say that a reaction has actually taken place?
- Can you correlate your observations for the Activities 3.9, 3.10 and 3.11?
- Write a balanced chemical equation for the reaction that has taken place. Name the type of reaction.
Ans:
- Test Tube 1: Copper wire in iron(II) sulphate (FeSO₄) solution.
- Test Tube 2: Iron nail in copper(II) sulphate (CuSO₄) solution.
Observations (After 20 Minutes):
- Test Tube 1 (Cu in FeSO₄): No visible change. The copper wire remains unchanged, and the solution retains its pale green colour.
- Test Tube 2 (Fe in CuSO₄): A reddish-brown deposit of copper forms on the iron nail, and the blue colour of the copper sulphate solution fades (becomes paler or colourless).
Reaction Occurrence: - Reaction occurs in Test Tube 2 (iron in copper sulphate).
- Basis for Reaction: The formation of a reddish-brown copper deposit on the iron nail and the fading of the blue colour of CuSO₄ solution indicate a displacement reaction. Iron displaces copper from CuSO₄ because iron is more reactive.
Correlation with Activities 3.9, 3.10, 3.11:
- Activity 3.9 (Oxygen): Iron reacts with oxygen (filings burn), while copper only forms an oxide layer, indicating iron is more reactive.
- Activity 3.10 (Water): Iron reacts with steam, while copper does not, confirming iron’s higher reactivity.
- Activity 3.11 (Acids): Iron reacts with dilute HCl, producing hydrogen, while copper does not, further showing iron’s greater reactivity.
- Conclusion: Iron is consistently more reactive than copper across these tests, supporting the displacement observed here.
Chemical Equation: Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
Type of Reaction: Single Displacement Reaction (a more reactive metal, iron, displaces a less reactive metal, copper, from its salt solution).
Conclusion: Iron is more reactive than copper, as per the reactivity series.
Activity 3.13: Properties of Ionic Compounds
- Take samples of sodium chloride, potassium iodide, barium chloride or any other salt from the science laboratory.
- What is the physical state of these salts?
- Take a small amount of a sample on a metal spatula and heat directly on the flame (Fig. 3.7). Repeat with other samples.
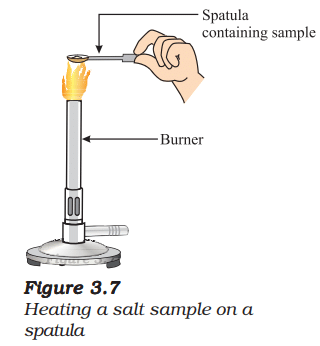
- What did you observe? Did the samples impart any colour to the flame? Do these compounds melt?
- Try to dissolve the samples in water, petrol and kerosene. Are they soluble?
- Make a circuit as shown in Fig. 3.8 and insert the electrodes into a solution of one salt. What did you observe? Test the other salt samples too in this manner.
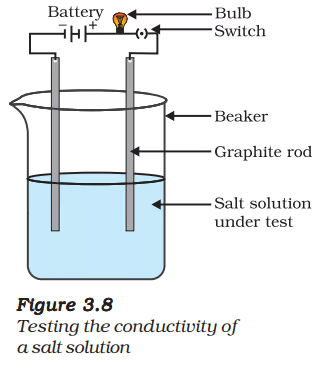
- What is your inference about the nature of these compounds?
Ans:
Salts Tested: Sodium chloride (NaCl), potassium iodide (KI), barium chloride (BaCl₂).
Physical State: All are solids at room temperature, appearing as white or colourless crystalline powders.
Heating on Flame:
- Sodium Chloride: Imparts a golden-yellow flame colour. Does not melt easily (high melting point, ~1074 K).
- Potassium Iodide: Imparts a violet flame colour. Does not melt easily (high melting point, ~954 K).
- Barium Chloride: Imparts a green flame colour. Does not melt easily (high melting point, ~1045 K).
- Observation: The flame colours are due to the excitation of metal ions (Na⁺, K⁺, Ba²⁺). The compounds do not melt under a Bunsen burner flame due to their high melting points.
Solubility:
- In Water: All salts (NaCl, KI, BaCl₂) are soluble, forming clear solutions.
- In Petrol/Kerosene: All salts are insoluble, as ionic compounds do not dissolve in non-polar solvents.
Conductivity Test: - Setup: Electrodes are inserted into an aqueous solution of each salt, connected to a circuit with a bulb.
- Observation: The bulb glows for all salt solutions (NaCl, KI, BaCl₂), indicating that the solutions conduct electricity.
- Explanation: Ionic compounds dissociate into ions in water (e.g., NaCl → Na⁺ + Cl⁻), which move and carry current.
Inference About Nature: These compounds are ionic compounds (electrovalent). Their properties include:
- Solid and Hard: Due to strong electrostatic forces between ions.
- High Melting/Boiling Points: Require significant energy to break ionic bonds.
- Soluble in Water, Insoluble in Non-Polar Solvents: Ions interact with polar water molecules.
- Conduct Electricity in Solution/Molten State: Due to free-moving ions, but not in solid state due to fixed ion positions.
Activity 3.14: Investigating Conditions for Iron Rusting
- Take three test tubes and place clean iron nails in each of them.
- Label these test tubes A, B and C. Pour some water in test tube A and cork it.
- Pour boiled distilled water in test tube B, add about 1 mL of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
- Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture, if any, from the air. Leave these test tubes for a few days and then observe (Fig. 3.13).
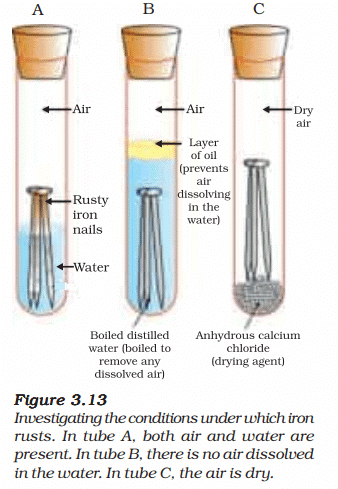
Ans:
- Test Tube A: Iron nails in water, exposed to air (oxygen dissolved in water).
- Test Tube B: Iron nails in boiled distilled water with an oil layer (no dissolved oxygen, as boiling removes air, and oil prevents air re-entry).
- Test Tube C: Iron nails with anhydrous calcium chloride (dry air, no moisture).
Observations (After a Few Days):
- Test Tube A: Iron nails show rust (brown flaky coating of iron(III) oxide, Fe₂O₃·nH₂O).
- Test Tube B: No rust; nails remain shiny (no oxygen available for rusting).
- Test Tube C: No rust; nails remain shiny (no moisture available for rusting).
Conclusion: Rusting of iron requires both moisture (water) and oxygen. Test Tube A has both, leading to rust. Test Tubes B and C lack either oxygen or moisture, preventing rust. - Reaction for Rusting: 4Fe(s) + 3O₂(g) + 2nH₂O(l) → 2Fe₂O₃·nH₂O(s) (rust)
Explanation: Rusting is an electrochemical process where iron oxidizes in the presence of water and oxygen, forming hydrated iron(III) oxide.
|
80 videos|569 docs|80 tests
|
FAQs on NCERT Based Activity: Metal and Non-metals - Science Class 10
| 1. What is metallic lustre and how can it be observed in metals? |  |
| 2. How is the hardness of metals tested? |  |
| 3. What is malleability in metals, and how can it be tested? |  |
| 4. Why is ductility important in metals, and how can it be observed in daily life? |  |
| 5. How do metals react with water and dilute hydrochloric acid in experiments? |  |
















