NCERT Exemplar: Alcohols, Phenols & Ethers | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Multiple Choice Questions -I |

|
| Multiple Choice Questions -II |

|
| Short Answer Type Questions |

|
| Matching Type Questions |

|
| Assertion and Reason Type Questions |

|
| Long Answer Type Questions |

|
Multiple Choice Questions -I
Q.1. Monochlorination of toluene in sunlight followed by hydrolysis with aq. NaOH yields.
(1) o-Cresol
(2) m-Cresol
(3) 2, 4-Dihydroxytoluene
(4) Benzyl alcohol
Ans. (4)
Solution.
Monochlorination of toluene in sunlight gives benzyl chloride. On hydrolysis with aq. NaOH, benzyl chloride shows nucleophilic substitution reaction to give benzyl alcohol.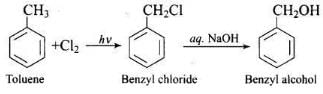
Q.2. How many alcohols with molecular formula C4H10O are chiral in nature?
(1) 1
(2) 2
(3) 3
(4) 4
Ans. (1)
Solution.
Only one alcohol contains chiral carbon atom.
(i) CH3CH2CH2CH2OH
(ii)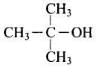
(iii)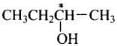
Only (iii) is chiral in nature.
Q.3. What is the correct order of reactivity of alcohols in the following reaction?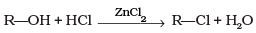
(1) 1° > 2° > 3°
(2) 1° < 2° > 3°
(3) 3° > 2° > 1°
(4) 3° > 1° > 2°
Ans. (3)
Solution.
The given reaction is nucleophilic substitution reaction in which -OH group is replaced by -Cl. Tertiary alcohols, when react with HCl in presence of ZnCl2, form tertiary carbocation. This intermediate 3° carbocation is more stable than 2° carbocation as well as 1° carbocation. The higher the stability of intermediate, the higher will be the reactivity of reactant molecule.
So, the order of reactivity of alcohols in the given reaction is 3° > 2° > 1°.
Q.4. CH3CH2OH can be converted into CH3CHO by ________.
(1) Catalytic hydrogenation
(2) Treatment with LiAlH4
(3) Treatment with pyridinium chlorochromate
(4) Treatment with KMnO4
Ans. (3)
Solution.
Alcohols are oxidized to aldehydes and finally to acids.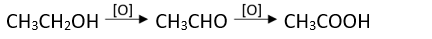
Oxidations can be be stopped at aldehyde stage by using pyridinium chlorochromate
(CrO3C5H5N.HCl).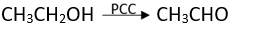
Q.5. The process of converting alkyl halides into alcohols involves _________.
(1) Addition reaction
(2) Substitution reaction
(3) Dehydrohalogenation reaction
(4) Rearrangement reaction
Ans. (2)
Solution.
Conversion of alkyl halides into alcohols involves substitution reaction.
Q.6. Which of the following compounds is aromatic alcohol?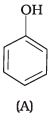
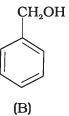
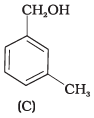
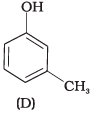
(1) A, B, C, D
(2) A, D
(3) B, C
(4) A
Ans. (3)
Solution.
Compound (A) i.e., phenol and compound (D) i.e., a derivative of phenol cannot be considered as aromatic alcohol. As phenol is also known as, carbolic acid cannot be considered as aromatic alcohol.
Compound (B) and (C), -OH group is bonded to sp3 hybridised carbon which in turn is bonded to benzene ring.
Q.7. Give IUPAC name of the compound given below.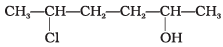
(1) 2-Chloro-5-hydroxyhexane
(2) 2-Hydroxy-5-chlorohexane
(3) 5-Chlorohexan-2-ol
(4) 2-Chlorohexan-5-ol
Ans. (3)
Solution.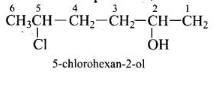
Q.8. IUPAC name of m -cresol is _________.
(1) 3-methylphenol
(2) 3-chlorophenol
(3) 3-methoxyphenol
(4) Benzene-1,3-diol
Ans. (1)
Solution.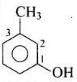
-OH is functional group and -CH3 is substituent.
IUPAC name: 3-methylphenol
Q.9. IUPAC name of the compound is ___________.
(1) 1-methoxy-1-methylethane
(2) 2-methoxy-2-methylethane
(3) 2-methoxypropane
(4) Isopropylmethyl ether
Ans. (3)
Solution.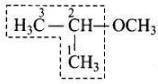 IUPAC name of the above compound is 2-methoxypropane.
IUPAC name of the above compound is 2-methoxypropane.
Q.10. Which of the following species can act as the strongest base?
(1) Θ OH
(2) Θ OR
(3) Θ O C6H5
(4)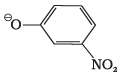
Ans. (2)
Solution.
Weakest acid has the strongest conjugate base. Since R-OH is the weakest acid, therefore, RO is the strongest base.
Q.11. Which of the following compounds will react with sodium hydroxide solution in water?
(1) C6H5OH
(2) C6H5CH2OH
(3) (CH3)3COH
(4) C2H5OH
Ans. (1)
Solution.
Phenol being more acidic reacts with sodium hydroxide solution in water to give sodium phenoxide which is resonance stabilized.
Alcohols are very weak acids.
C6H5OH + NaOH → C6H5ONa + H2O
Q.12. Phenol is less acidic than __________.
(1) Ethanol
(2) o-nitrophenol
(3) o-methylphenol
(4) o-methoxyphenol
Ans. (2)
Solution.
In o-nitrophenol, nitro group is present at ortho position. Presence of electron withdrawing group at ortho position increases the acidic strength. On the other hand, in o-methylphenol and in o-methoxyphenol and in o-methoxyphenol, electron releasing group (-CH3-OCH3).
Presence of these groups at ortho or para positions of phenol decreases the acidic strength of phenols. So, phenol is less acidic than o-nitrophenol.
Q.13. Which of the following is most acidic?
(1) Benzyl alcohol
(2) Cyclohexanol
(3) Phenol
(4) m-Chlorophenol
Ans. (4)
Solution.
Alcohols are less acidic than phenol. Further electron withdrawing group (like – Cl) increases the acidity of phenol, therefore, m-chlorophenol is most.
Q.14. Mark the correct order of decreasing acid strength of the following compounds.
(a)
(b)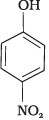
(c)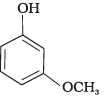
(d)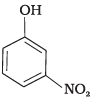
(e)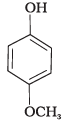
(1) e > d > b > a > c
(2) b > d > a > c > e
(3) d > e > c > b > a
(4) e > d > c > b > a
Ans. (2)
Solution.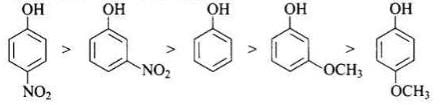
-NO2 is an electron withdrawing group which increases the acidity of phenol and the effect is more pronounced at ortho and para positions. Similarly methoxy group is an electron releasing group which decreases the acidity of phenol and the effect is more pronounced at ortho and para positions.
Q.15. Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
(a)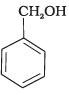
(b)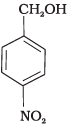
(c)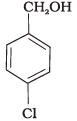
(1) a < b < c
(2) b < a < c
(3) b < c < a
(4) c < b < a
Ans. (3)
Solution.
All three benzyl alcohols react with HBr/HCl through the formation of intermediate carbocation. The more stable the carbocation, the more reactive is the alcohol. The electron releasing groups i.e. NO2, Cl decrease the stability of the carbocation. Since -NO2 group is stronger electron withdrawing than -Cl group, therefore, stability of carbocation increases in the order: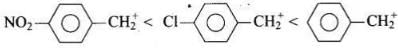
Therefore, the reactivity of benzyl alcohols increases in the order: (b) < (c)<(a)
Q.16. Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(1) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol
(2) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(3) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol
(4) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Ans. (1)
Solution.
Boiling point increases with increase in molecular mass of the alcohols. Among isomeric alcohols 1° alcohols have higher boiling points than 2° alcohols. Thus, correct order is:
Propan-l-ol < butan-2-ol < butan-l-ol < pentan-l-ol.
Multiple Choice Questions -II
Note : In the following questions two or more options may be correct.
Q.17. Which of the following are used to convert RCHO into RCH2OH?
(1) H2/Pd
(2) LiAlH4
(3) NaBH4
(4) Reaction with RMgX followed by hydrolysis
Ans. (1, 2, 3)
Solution.
Conversion of aldehyde into alcohol is a reduction reaction. This reduction can be carried out by adding hydrogen in presence of finely divided metal catalyst such as platinum, palladium or nickel.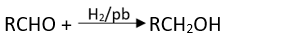
It can also be prepared by using NaBH4 and LiAlH4 as a reducing agent.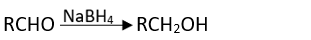
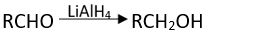
Reaction of RMgX with any aldehyde other than methanal gives secondary alcohols not the primary alcohols.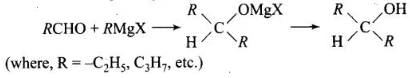
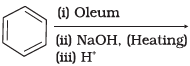
Q.18. Which of the following reactions will yield phenol?
(1)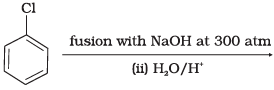
(2)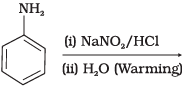
(3)
(4)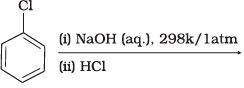
Ans. (1, 2, 3)
Solution.
Haloarene requires extreme conditions for nucleophilic substitution reaction. Chlorobenzene does not undergo hydrolysis on treatment with aqueous NaOH at 298 K and 1 atm.
Q.19. Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(1) CrO3 in anhydrous medium.
(2) KMnO4 in acidic medium.
(3) Pyridinium chlorochromate.
(4) Heat in the presence of Cu at 573K.
Ans. (1, 3, 4)
Solution.
CrO3 in anhydrous medium, PCC and heating in presence of Cu (dehydrogenation) will oxidize primary alcohols to aldehydes. KMnO4 will further oxidize it to acid.
Q.20. Phenol can be distinguished from ethanol by the reactions with _________.
(1) Br2/water
(2) Na
(3) Neutral FeCl3
(4) All the above
Ans. (1, 3)
Solution.
Phenol reacts with bromine water to give a colourless tribromo derivative and gives a violet coloured complex with FeCl3. Ethanol does not give these reactions.
Q.21. Which of the following are benzylic alcohols?
(1) C6H5—CH2—CH2OH
(2) C6H5—CH2OH
(3) 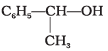
(4)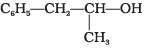
Ans. (2, 3)
Solution.
In benzylic alcohols, the -OH group is attached to a sp3 hybridised carbon atom next to an aromatic ring. In compounds (1) and (4), the -OH group is attached to a sp3 hybridised carbon atom but this carbon is not attached to the benzene ring.
On the other hand, in compounds (1) and (3), the -OH group is attached to a sp3 hybridised carbon atom next to an aromatic ring.
Short Answer Type Questions
Q.22. What is the structure and IUPAC name of glycerol?
Ans.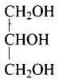
IUPAC name of glycerol is Propane - 1,2,3-triol.
Q.23. Write the IUPAC name of the following compounds.
(a)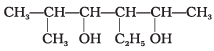
(b)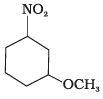
Ans.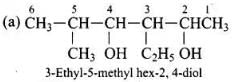
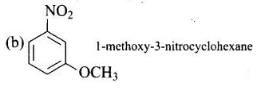
Q.24. Write the IUPAC name of the compound given below.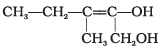 Ans.
Ans.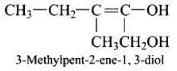
Q.25. Name the factors responsible for the solubility of alcohols in water.
Ans. Alcohols are soluble in water because of hydrogen bonding with water molecules.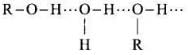
The solubility decreases with increase in size of alkyl or aryl group.
Q.26. What is denatured alcohol?
Ans. Alcohol is made unfit for drinking by mixing some copper sulphate and pyridine to it. This process is called denaturation of alcohol and alcohol mixed with copper sulphate and pyridine is called denatured alcohol.
Q.27. Suggest a reagent for the following conversion. Ans. CrO3, pyridine and HC1 (Pyridinium chlorochromate) C3H5 N HCr03Cl.
Ans. CrO3, pyridine and HC1 (Pyridinium chlorochromate) C3H5 N HCr03Cl.
Q.28. Out of 2-chloroethanol and ethanol which is more acidic and why?
Ans. 2-Chloroethanol is more acidic, due to -I effect of chlorine atom.
Q.29. Suggest a reagent for conversion of ethanol to ethanal.
Ans. CrO3, pyridine and HCl (Pyridinium chlorochromate, PCC) in CH2C12.
Q.30. Suggest a reagent for conversion of ethanol to ethanoic acid.
Ans. Any strong oxidising agent e.g., acidified K2Cr2O7 or KMnO4.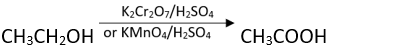
Q.31. Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
Ans. Ortho nitrophenol is much more volatile in steam due to chelation. Intramolecular hydrogen bonding is present in o-nitrophenol and intermolecular hydrogen bonding in p-nitrophenol.
Q.32. Out of o-nitrophenol and o-cresol which is more acidic?
Ans. Nitro group is electron is electron withdrawing while -CH3 group is electron releasing.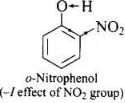
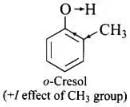
As a result, O – H bond in o-nitrophenol is much weaker than the O – H bond in o-cresol and hence o-nitrophenol is much more acidic than o-cresol.
Q.33. When phenol is treated with bromine water, white precipitate is obtained. Give the structure and the name of the compound formed.
Ans.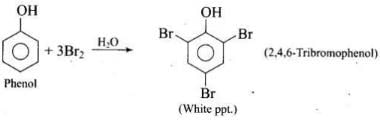
Q.34. Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
Ans. Increasing order of acidity.
o-Cresol < Phenol < o-Nitrophenol
In cresol,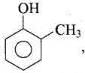 the electron donating group (-CH3) gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron withdrawing (-NO2) group withdraws electrons and disperses the -ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
the electron donating group (-CH3) gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron withdrawing (-NO2) group withdraws electrons and disperses the -ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
Q.35. Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
Ans. Decreasing order of reactivity of sodium metal is: 1° > 2° > 3°
Alcohols react with sodium metal to form alkoxides and hydrogen is liberated:
R - O - H + Na → RO - Na+ + 1/2 H2
The order of reactivity of alcohols is primary > secondary > tertiary. This can be explained on the basis of cleavage of O – H bond. The alkyl groups are electron releasing groups (+1 effect) and they increase the electron density around the oxygen. As a result, the electrons of O – H bond cannot be withdrawn strongly towards oxygen and O – H remains strong. Therefore, greater is the number, of alkyl groups present, smaller will be reactivity of alcohol.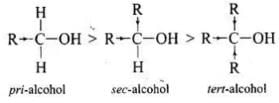
Q.36. What happens when benzene diazonium chloride is heated with water?
Ans.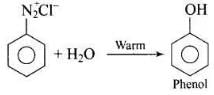
Q.37. Arrange the following compounds in decreasing order of acidity.
H2O, R-OH, HC ≡ CH
Ans. Decreasing order of acidify is: H2O>R-OH>HC = CH
Q.38. Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
Ans. Sucrose is converted to glucose and fructose in the presence of an enzyme, invertase or sucrose. Glucose and fructose undergo fermentation in the presence of another enzyme, zymase. Both these enzymes are present in yeast.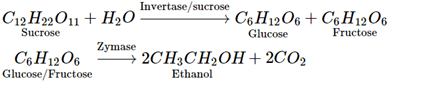
Q.39. How can propan-2-one be converted into tert- butyl alcohol?
Ans.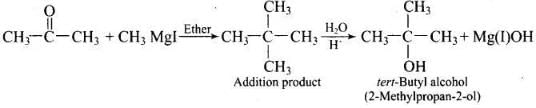
Q.40. Write the structures of the isomers of alcohols with molecular formula C4H10O.Which of these exhibits optical activity?
Ans. Isomers of alcohols (C4H10O) are:
(a)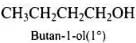
(b)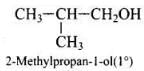
(c)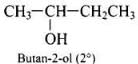
(d)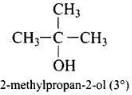
Butan-2-ol is optically active.
Q.41. Explain why is OH group in phenols more strongly held as compared to OH group in alcohols.
Ans. In phenol, oxygen atom is attached to sp2-hybridised carbon atom while in alcohol, it is attached to sp3-hybridised carbon atom. The bond formed between oxygen and sp2-hybridised carbon is more strongly held then that formed between oxygen and sp3-hybridised carbon.
Q.42. Explain why nucleophilic substitution reactions are not very common in phenols.
Ans.The C – O bond in phenols has some double bond character due to resonance and hence cannot be easily cleaved by nucleophiles. So, nucleophilic substitution reactions are not very common in phenols and they give many electrophilic substitution reactions.
Q.43. Preparation of alcohols from alkenes involves the electrophilic attack on alkene carbon atom. Explain its mechanism.
Ans. The mechanism of acid catalysed addition of water (hydration) to alkenes involves the following three steps:
Step 1: Electrophilic attack by hydronium ion (H3O+) on alkene gives an intermediate, carbocation.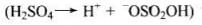
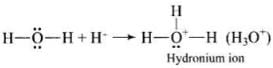
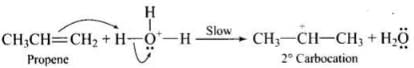
Step 2: Nucleophilic attack by water on carbocation to yield protonated alcohol.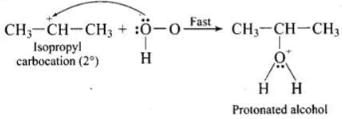
Step 3: Deprotonation (loss of proton) to form an alcohol.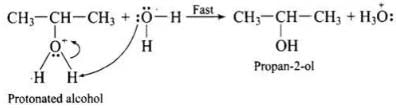
Q.44. Explain why is O = C = O nonpolar while R—O—R is polar.
Ans. O = C = O molecule is linear so that the polarities of two C – O bonds get cancelled and the molecule is linear.
Ethers have structures similar to water and have angular or bent structure. Therefore, the polarity of two R – O groups does not get cancelled and these have net dipole moment. Thus, R – O – R is polar.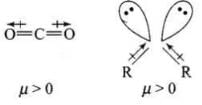
Q.45. Why is the reactivity of all the three classes of alcohols with conc. HCl and ZnCl2 (Lucas reagent) different?
Ans. The alcohol combine with HCl to form protonated alcohol. The positive charge on oxygen weakens the C – O bond leading to its cleavage.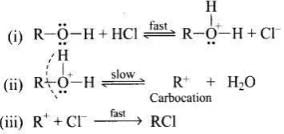
The rate determining step in the above mechanism is (ii), which is a slow step reaction. The stability of carbocation will determine the reactivity of the reaction. Since the order of stability of carbocation is: Pri. < sec. < tert. Hence the order of formation of alkyl halide in the above reaction is pri. < sec. < tert.
Q.46. Write steps to carry out the conversion of phenol to aspirin.
Ans.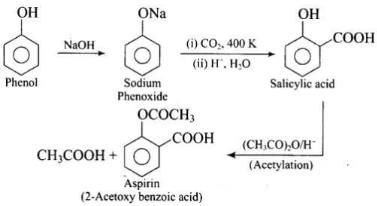
Q.47. Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
Ans. Phenol is more easily nitrated than benzene because the —OH group in phenol increases the electron density at o- and p-positions in benzene ring by +R effect. Nitration which is an electrophilic substitution reaction takes place more readily where the electron density is more.
Q.48. In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Ans. Phenoxide ion is more reactive than phenol towards electrophilic substitution. The negative charge on oxygen is transferred to benzene through resonance. This helps in the attachment of CO2 which is a weak electrophile to the benzene ring finally giving salicylic acid.
Q.49. Dipole moment of phenol is smaller than that of methanol. Why?
Ans. In phendl, C – O bond is less polar due to electron withdrawing effect of benzene ring, whereas in methanol C – O bond is more polar due to electron releasing effect of—CH3 group. Hence, the dipole moment of phenol (1.54 D) is smaller than that of methanol (1.71 D).
Q.50. Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
Ans. In tert-butyl halides, the elimination reaction is favoured over substitution. So, alkene is the only reaction product formed and not the ether.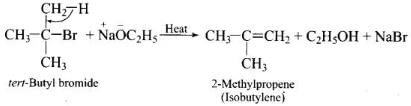
Q.51. Why is the C—O—H bond angle in alcohols slightly less than the tetrahedral angle whereas the C—O—C bond angle in ether is slightly greater?
Ans. The C – O – H bond angle in alcohols is slightly less than the tetrahedral angle (109.5°) because of larger repulsions between the lone pairs of electrons. For example in methanol C – O – H bond angle is 108.9°.
In ethers, the C – O – C bond angle is slightly greater than tetrahedral angle. For example in dimethyl ether C – O – C bond angle is 111.7°. The larger bond angle in ethers may be because of greater repulsions between bulkier alkyl groups as compared to one H in alcohols.
Q.52. Explain why low molecular mass alcohols are soluble in water.
Ans. The lower members of alcohols are highly soluble in water but the solubility decreases with increase in molecular weight. The solubility of lower alcohols in water is due to the formation of hydrogen bonds between alcohols and water molecules.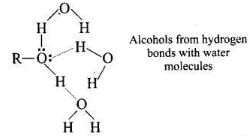
Q.53. Explain why p-nitrophenol is more acidic than phenol.
Ans. The electron withdrawing group (-NO2), withdraws electrons and disperses the negative charge. Therefore, -NO2 group stabilizes the phenoxide ion. Hence p-nitrophenol is more acidic than phenol.
Q.54. Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Ans. Ethers have low polarity and as a result do not show any association by intermolecular hydrogen bonding. Therefore, ethers have low boiling points and lower than that of isomeric alcohols and almost same as those of alkanes of comparable molecular masses.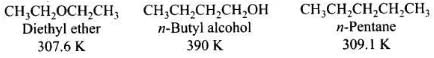
The large difference in boiling points of alcohols and ethers is due to the presence of hydrogen bonding in alcohols.
Q.55. The carbon-oxygen bond in phenol is slightly stronger than that in methanol.Why?
Ans. This can be explained as under:
(i) In phenol, the conjugation of unshared electron pairs over oxygen with aromatic ring results in partial double bond character in C – O bond.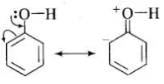
In methanol, no such conjugation (resonance) is possible.
(ii) In phenol, oxygen is attached to sp2 hybridised carbon while in methanol, oxygen attached to sp2 hybridised carbon. An sp2 hybridised carbon is more electronegative (because of greater 5-character) than sp3 hybridised carbon atom. Therefore, the bond between oxygen and sp2 hybridised carbon is more stable than the bond between oxygen and sp2, hybridised orbital.
Q.56. Arrange water, ethanol and phenol in increasing order of acidity and give reason for your answer.
Ans. Increasing order of acidity is: ethanol < water < phenol.
This is because the phenoxide ion obtained after the removal of H+ is resonance stabilised, while the ethoxide ion obtained after the removal of H+ is destabilised by +1 effect of ethyl group. Thus phenol is a stronger acid than ethanol.
Now, ethanol is a weaker acid than water because the electron releasing ethyl group increases the ethanol density on oxygen and consequently the proton will not be released easily. There is no such effect is water.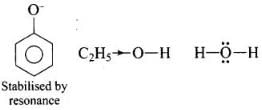
Matching Type Questions
Note : Match the items of Column I and Column II in the following questions.
Q.57. Match the structures of the compounds given in Column I with the name of the compounds given in Column II.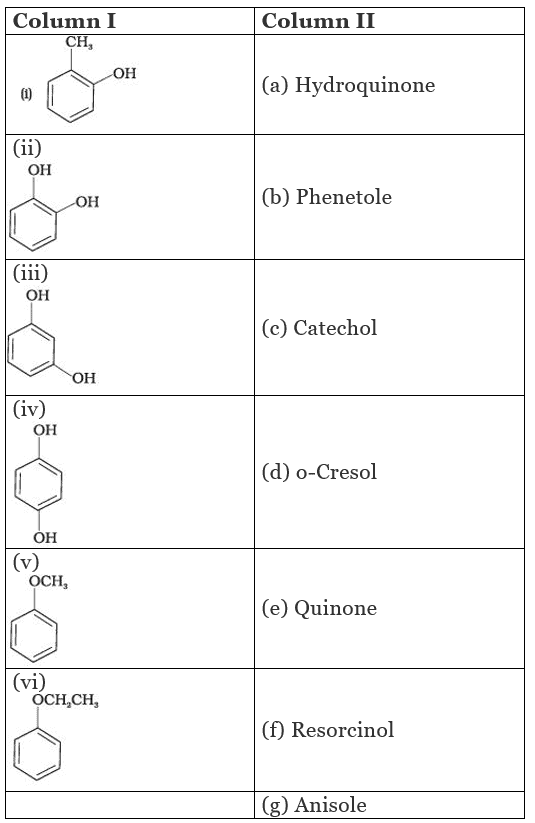
Ans. (i → d), (ii → c), (iii → f), (iv → a), (v → g), (vi → b)
Solution.
(i) Cresols are organic compounds which are methyl phenols. There are three forms of cresol: o-cresol, p-cresol and w-cresol.
(ii) Catechol is also known as pyrocatechol. Its IUPAC name is 1, 2-dihydroxbenzene. It is used in the production of pesticides, perfumes and pharmaceuticals.
(iii) Its IUPAC name is 1, 3-dihydroxybenzene. Resorcinol is used to treat acne, seborrheic dermatitis and other skin disorder.
(iv) Hydroquinone is also known as quinol. Its IUPAC name is 1, 4-dihydroxybenzene. It is a white granular solid.lt is a good reducing agent.
(v) Anisole or methoxy benzene, is a colourless liquid with a smell reminiscent of anise seed.
(vi) Phenetole is an organic compound. It is also known as ethylphenyl ether. It is volatile in nature and its vapour are explosive in nature.
Q.58. Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.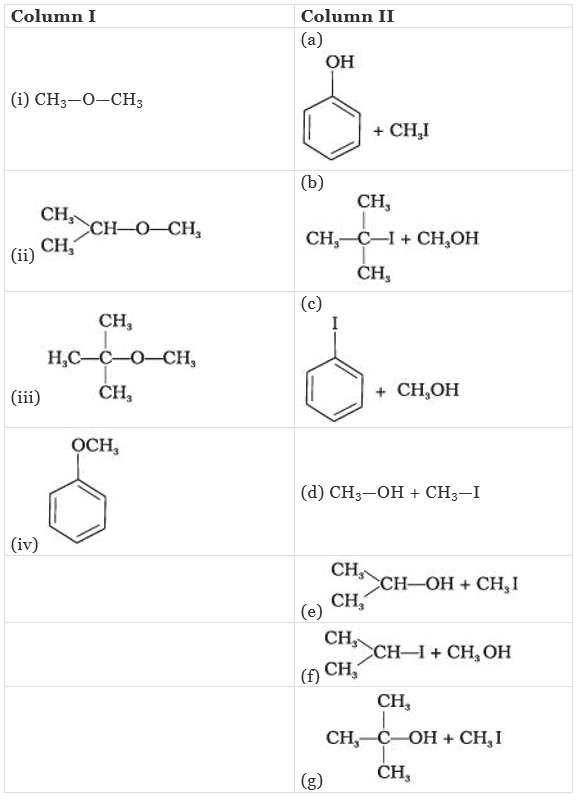
Ans. (i → d), (ii → e), (iii → b), (iv → a)
Solution.
(i) CH3 – O – CH3 is a symmetrical ether so the products are CH3I and CH2OH.
(ii) In (CH3)2CH – O – CH3 unsymmetrical ether, one alkyl group is primary while another is secondary. So, it follows SN2mechanism. Thus, the halide ion attacks the smaller alkyl group and the products are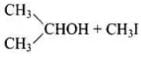
(iii) In this case, one of the alkyl group is tertiary and the other is primary. It follows SN1 mechanism and halide ion attacks the tertiary alkyl group and the products are (CH3)3C-I and CH3OH.
(iv) Here, the unsymmetrical ether is alkyl aryl ether. In this ether 0-CH3 bond is weaker than 0-C6H5 bond which has partial double bond character due to resonance. So, the halide ion attacks on alkyl group and the products are C6H5-OH and CH3I.
Q.59. Match the items of column I with items of column II.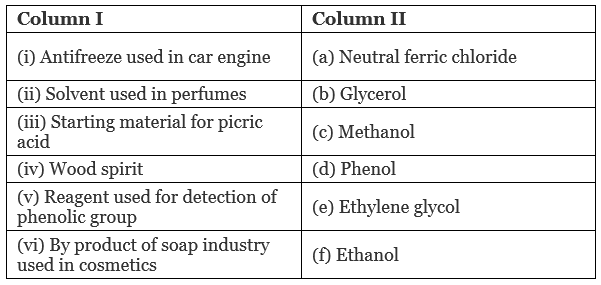
Ans. (i → e), (ii → f), (iii → d), (iv → c), (v → a), (vi → b)
Solution.
(i) IUPAC name of ethylene glycol is ethane-1, 2-diol. It is primarily used as raw material in the manufacturing of polyester fibers and fabric industry. A small percentage of it is used in antifreeze formulations.
(ii) Ethanol is a good solvent for fatty and waxy substances. Fats and waxes provide odour to the perfumes. Apart from being a good solvent, it is less irritating to the skin. So, it is used in perfumes.
(iii) Phenol is converted into picric acid (2, 4, 6-trinitro-phenol) by the reaction of phenol with cone. HNO3.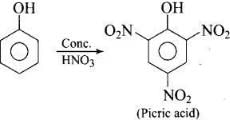 (iv) Methanol, CH3OH is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(iv) Methanol, CH3OH is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(v) Neutral ferric chloride give purple/red colour when treated with phenols. It is the reagent used for detection of phenolic group.
(vi) Soaps are prepared by the reactions of fatty acid with NaOH.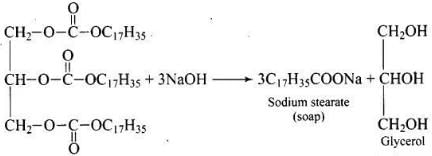
The glycerol (propane-1,2,3-triol) is the byproduct of soap industry and used in cosmetics.
Q.60. Match the items of column I with items of column II.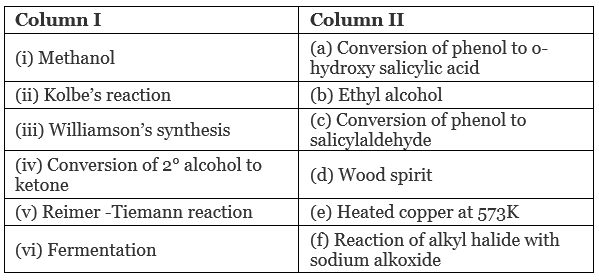
Ans. (i → d), (ii → a), (iii → f), (iv → e), (v → c), (vi → b)
Solution.
(i) Methanol is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(ii) In Kolbe’s reaction, 2-hydroxy benzoic acid (salicylic acid) is prepared by the reaction of phenol with CO2 gas.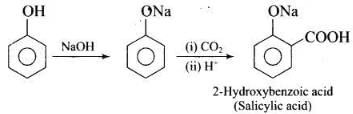 (iii) Williamson's synthesis is an important method for the preparation of ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.
(iii) Williamson's synthesis is an important method for the preparation of ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.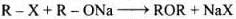
(iv) When a 2° alcohol is allowed to pass over heated copper at 573 K, dehydrogenation takes place and an ketone is formed.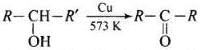
(v) On treating phenol with chloroform in the presence of NaOH, an aldehydic group is introduced at ortho position of benzene ring.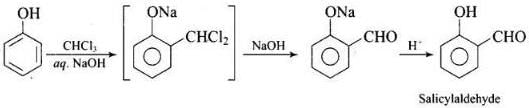
(vi) Ethanol is prepared by the fermentation of sugars.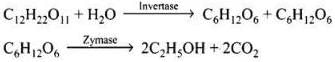
Assertion and Reason Type Questions
Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Q.61. Assertion : Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol
Reason : Addition of water in acidic medium proceeds through the formation of primary carbocation.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (2)
Solution.
Addition of water to but-1-ene in acidic medium yields butan-2-ol.
Addition of water proceeds through formation of secondary carbocation.
Q.62. Assertion : p-nitrophenol is more acidic than phenol.
Reason : Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (1)
Solution.
p-Nitrophenol is more acidic than phenol because nitro group stabilizes phenoxide ion by dispersal of negative charge.
Q.63. Assertion : IUPAC name of the compound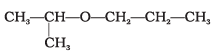 is 2-Ethoxy-2-methylethane.
is 2-Ethoxy-2-methylethane.
Reason : In IUPAC nomenclature, ether is regarded as hydrocarbon derivative in which a hydrogen atom is replaced by —OR or —OAr group [where R = alkyl group and Ar = aryl group]
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (4)
Solution.
IUPAC name of the compound is 2-propoxypropane.
Q.64. Assertion : Bond angle in ethers is slightly less than the tetrahedral angle.
Reason : There is a repulsion between the two bulky (—R) groups.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (4)
Solution.
Bond angle in ethers is slightly more than the tetrahedral angle due to repulsion between two bulky alkyl group.
Q.65. Assertion : Boiling points of alcohols and ethers are high.
Reason : They can form intermolecular hydrogen-bonding.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (2)
Solution.
Boiling points of alcohols are higher than ethers. Alcohols can form intermolecular hydrogen bonding whereas ethers cannot.
Q.66. Assertion : Like bromination of benzene, bromination of phenol is also carried out in the presence of Lewis acid.
Reason : Lewis acid polarises the bromine molecule.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (4)
Solution.
Bromination of phenol can be carried out in absence of Lewis acid.
Q.67. Assertion : o-Nitrophenol is less soluble in water than the m - and p-isomers.
Reason : m - and p- Nitrophenols exist as associated molecules.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (5)
Solution.
In o-nitrophenol there is intramolecular H-bonding.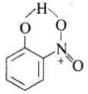 Thus, o-nitrophenol does not form H-bond with H2O but m-nitrophenol and p-nitrophenol form H-bonds with H2O. Also, due to intermolecular H-bonding m-nitrophenol and p-nitrophenol exist as associated molecules.
Thus, o-nitrophenol does not form H-bond with H2O but m-nitrophenol and p-nitrophenol form H-bonds with H2O. Also, due to intermolecular H-bonding m-nitrophenol and p-nitrophenol exist as associated molecules.
Q.68. Assertion : Ethanol is a weaker acid than phenol.
Reason : Sodium ethoxide may be prepared by the reaction of ethanol with aqueous NaOH.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (3)
Solution.
Phenol is stronger acid than ethanol as phenoxide ion is stabilized by resonance whereas no such stabilization occurs in ethoxide ion. Sodium ethoxide can be prepared by reaction of ethanol with sodium.
Q.69. Assertion : Phenol forms 2, 4, 6 – tribromophenol on treatment with Br2 in carbon disulphide at 273K.
Reason : Bromine polarises in carbon disulphide.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (2)
Solution.
Phenol forms 2, 4, 6-tribromophenol on treatment with bromine in water. In phenols, the polarization of bromine takes place even in the absence of Lewis acid.
Q.70. Assertion : Phenols give o- and p-nitrophenol on nitration with conc. HNO3 and H2SO4 mixture.
Reason : —OH group in phenol is o–, p– directing.
(1) Assertion and reason both are correct and reason is correct explanation of assertion.
(2) Assertion and reason both are wrong statements.
(3) Assertion is correct statement but reason is wrong statement.
(4) Assertion is wrong statement but reason is correct statement.
(5) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Ans. (4)
Solution.
Phenols give o, p-nitrophenol on nitration with dil. HNO3 and with conc.
• HNO3, 2,4, 6-trinitrophenol is formed.
Long Answer Type Questions
Q.71. Write the mechanism of the reaction of HI with methoxybenzene.
Ans. In case of alkyl aryl ethers, the products are always phenol and an alkyl halide because due to resonance C6H5 – O bond has partial double bond character.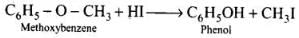
Mechanism:
Step -1: Ether molecule first gets protonated to give methylphenyl oxonium ion.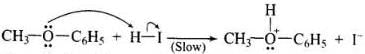
In this ion, the bond between O - CH3 is weaker than the bond between O - C6H5 which has partial double bond character. This partial double bond character is due to the resonance between the lone pair of electrons on the O-atom and the sp2 hybridised carbon atom of the phenyl group.
Step - 2: Protonated ether undergoes SN2 attack by I ion to give methyl iodide and phenol.
Q.72. (1) Name the starting material used in the industrial preparation of phenol.
(2) Write complete reaction for the bromination of phenol in aqueous and non aqueous medium.
(3) Explain why Lewis acid is not required in bromination of phenol?
Ans.
(1) Cumene (Isopropyl benzene) is the starting material used in industrial preparation of phenol.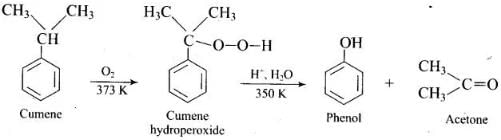
(2) Bromination of phenol
(i) in aqueous medium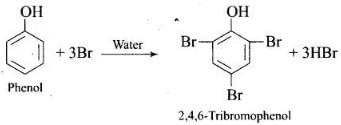
(ii) in non-aqueous medium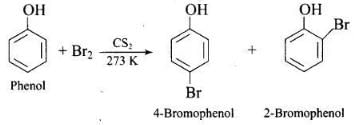
(c) Usual halogenation is carried out in the presence of Lewis acid, FeBr3 which polarises the halogen molecule. In case of phenol, the polarisation of bromine occurs even in the absence of Lewis acid. This is because of highly activating effect of -OH group on the benzene ring. The reaction follows: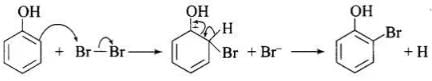
Note: In aqueous solution, phenol ionizes to give phenoxide ion. Due to the presence of the negative charge, the oxygen atom of the phenoxide ion donates electrons to the benzene ring to a large extent. As a result, the ring gets highly activated leading to the formation of trisubstituted product. On the other hand, in the non-polar solvents, the ionization of phenol does not occurs to a large extent. As a result, the -OH group donates electrons to the benzene ring only to a small extent. Consequently, the ring is activated slightly and, therefore, only monosubstitution occurs.
Q.73. How can phenol be converted to aspirin?
Ans. Phenol is converted into salicylic acid. The reaction is usually carried out by allowing sodium phenoxide to absorb carbon dioxide and then heating the product to 400 K and 4-7 atm pressure. First unstable intermediate is formed which undergoes a proton shift to form sodium salicylate. The subsequent acidification of sodium salicylate gives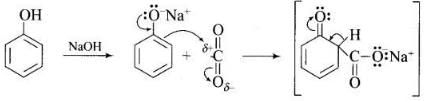
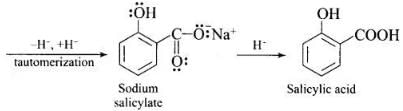
Then aspirin is obtained by acetylating salicyclic acid with acetic anhydride and conc. H2SO4.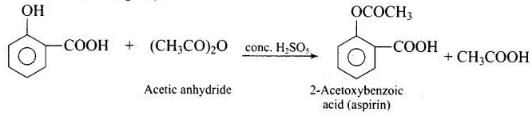
Q.74. Explain a process in which a biocatalyst is used in industrial preparation of a compound known to you.
Ans.
Biocatalysts are complex organic compounds which act as catalysts in reaction taking place in living organism. These biocatalysts (enzymes) are used in the manufacture of ethanol.
Ethanol from sugar solution (molasses): Molasses is a non-crystalline form of sugar obtained as the mother liquor after crystallisation of sugar from sugar solution. This contains about 50% sugar. It is diluted to about 10% solution and yeast is added and kept for about 2-3 days. Yeast supplies the enzymes invertase and zymase. The enzyme invertase hydrolyses sucrose to glucose and fructose. The enzyme zymase (found in yeast) converts glucose and fructose to ethanol.
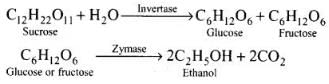
In wine making, grapes are the source of sugars and yeast. As grapes ripen, the quantity of sugar increases and the yeast grows on the skin of the grapes. When the grapes are crushed, sugar and enzyme come in contact and fermentation starts. Fermentation takes place under anaerobic conditions (i.e., in the absence of air). During fermentation CO2 is released.
The action of enzyme is inhibited when the concentration of alcohol exceeds 14%. If air enters the fermentation mixture, the O2 of the air oxidised ethanol to ethanoic acid which spoils the taste of alcoholic drinks and makes it sour.
|
75 videos|278 docs|78 tests
|
FAQs on NCERT Exemplar: Alcohols, Phenols & Ethers - Chemistry Class 12 - NEET
| 1. What are the main differences between alcohols, phenols, and ethers? |  |
| 2. How do alcohols behave as acids or bases? |  |
| 3. What are the methods for preparing ethers? |  |
| 4. What are some common uses of phenols in industry? |  |
| 5. How do the boiling points of alcohols compare to those of ethers? |  |
















