NCERT Exemplar: Biomolecules | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Multiple Choice Questions -I |

|
| Multiple Choice Questions -II |

|
| Short Answer Type Questions |

|
| Matching Type Questions |

|
| Long Answer Type Questions |

|
Multiple Choice Questions -I
Q.1. Glycogen is a branched chain polymer of α-D-glucose units in which chain is formed by C1 — C4 glycosidic linkage whereas branching occurs by the formation of C1 — C6 glycosidic linkage. Structure of glycogen is similar to __________.
(1) Amylose
(2) Amylopectin
(3) Cellulose
(4) Glucose
Ans. (2)
Solution.
Structure of glycogen is similar to amylopeptin. It is a branched chain polymer of α-D glucose units in which chain is formed by C1-C4 glycosidic linkage and branching occurs by C1-C6 glycosidic linkage.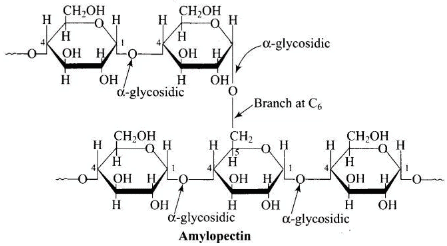
Q.2. Which of the following polymer is stored in the liver of animals?
(1) Amylose
(2) Cellulose
(3) Amylopectin
(4) Glycogen
Ans. (4)
Solution.
Glycogen is stored in the liver of animals.
Q.3. Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives _________.
(1) 2 molecules of glucose
(2) 2 molecules of glucose + 1 molecule of fructose
(3) 1 molecule of glucose + 1 molecule of fructose
(4) 2 molecules of fructose
Ans. (3)
Solution.
Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives one molecule of glucose and one molecule of fructose.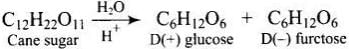
Note: Sucrose is a dextrorotatory sugar on hydrolysis produces a laevorotatory mixture, so known as invert sugar. Sucrose is a non-reducing sugar while maltose and lactose are reducing sugar.
Q.4. Which of the following pairs represents anomers?
(i)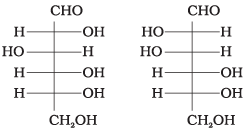
(ii)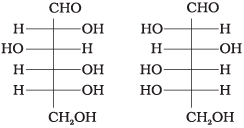
(iii)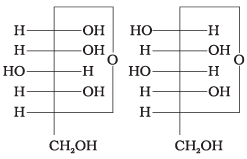
(iv)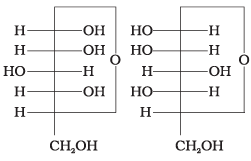
Ans. (c)
Solution.
The isomers which differ only in the configuration of the hydroxyl group at C—1 are called anomers and are referred to as α- and β-fonns.
Q.5. Proteins are found to have two different types of secondary structures viz. α-helix and β-pleated sheet structure. α-helix structure of protein is stabilised by :
(1) Peptide bonds
(2) Van der Waals forces
(3) Hydrogen bonds
(4) Dipole-dipole interactions
Ans. (3)
Solution.
α-helix structure of protein is stabilized by hydrogen bonds. A polypeptide chain forms all possible hydrogen bonds by twisting into a right handed helix with the -NH group of each amino acid residue hydrogen bonded to > C = O of an adjacent turn of helix.
Q.6. In disaccharides, if the reducing groups of monosaccharides i.e. aldehydic or ketonic groups are bonded, these are non-reducing sugars. Which of the following disaccharide is a non-reducing sugar?
(i)
(ii) 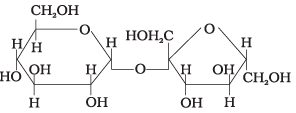
(iii)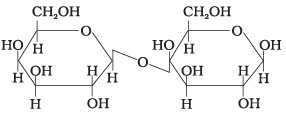
(iv) 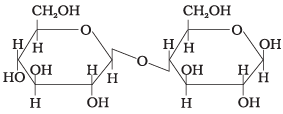
Ans. (2)
Solution.
This structure represents sucrose in which α-D glucose and β-D-fructose is attached to each other by C1 — C2 glycosidic linkage.
Since, reducing groups of glucose and fructose are involved in glycosidic bond formation, this is considered as non-reducing sugar.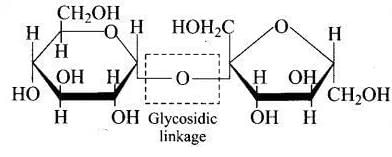
Q.7. Which of the following acids is a vitamin?
(1) Aspartic acid
(2) Ascorbic acid
(3) Adipic acid
(4) Saccharic acid
Ans. (2)
Solution.
Ascorbic acid is vitamin C. Aspartic acid is an amino acid. Adipic acid and saccharic acid are dicarboxylic acids.
Q.8. Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present?
(1) 5′ and 3′
(2) 1′ and 5′
(3) 5′ and 5′
(4) 3′ and 3′
Ans. (1)
Solution.
5′ and 3′ linkages are present between pentose sugars of nucleotides.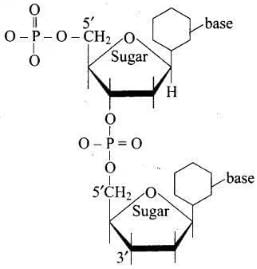
Q.9. Nucleic acids are the polymers of ______________.
(1) Nucleosides
(2) Nucleotides
(3) Bases
(4) Sugars
Ans. (2)
Solution.
Nucleic acids are polymers of nucleotides in which nucleic acids are linked together by phosphodiester linkage.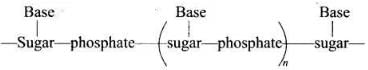
e.g., DNA, RNA, etc.,
Q.10. Which of the following statements is not true about glucose?
(1) It is an aldohexose.
(2) On heating with HI it forms n-hexane.
(3) It is present in furanose form.
(4) It does not give 2,4-DNP test.
Ans. (3)
Solution.
It is present in pyranose structure.
Q.11. Each polypeptide in a protein has amino acids linked with each other in a specific sequence. This sequence of amino acids is said to be ____________.
(1) Primary structure of proteins.
(2) Secondary structure of proteins.
(3) Tertiary structure of proteins.
(4) Quaternary structure of proteins.
Ans. (1)
Solution.
The sequence of amino acids in a polypeptide chain in called primary structure of proteins.
Q.12. DNA and RNA contain four bases each. Which of the following bases is not present in RNA?
(1) Adenine
(2) Uracil
(3) Thymine
(4) Cytosine
Ans. (3)
Solution.
DNA contains four bases adenine, guanine, thymine and cytosine. While RNA contains four bases adenine, uracil, guanine and cytosine. Thus, RNA does not contain thymine.
Hence, statement (c) is the correct choice.
Q.13. Which of the following B group vitamins can be stored in our body?
(1) Vitamin B1
(2) Vitamin B2
(3) Vitamin B6
(4) Vitamin B12
Ans. (4)
Solution.
Vitamin B12 can be stored in our body because it is insoluble in water.
Q.14. Which of the following bases is not present in DNA?
(1) Adenine
(2) Thymine
(3) Cytosine
(4) Uracil
Ans. (4)
Solution.
Uracil is not present in DNA, instead thymine is present.
Q.15. Three cyclic structures of monosaccharides are given below which of these are anomers.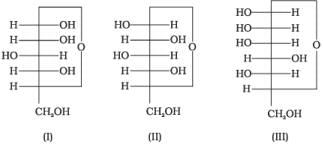
(1) I and II
(2) II and III
(3) I and III
(4) III is anomer of I and II
Ans. (1)
Solution.
Cyclic structures of monosaccharides which differ in structure at carbon-1 are known as anomers.
Here, I and II are anomer because they differ from each other at carbon-1 only.
Q.16. Which of the following reactions of glucose can be explained only by its cyclic structure?
(1) Glucose forms pentaacetate.
(2) Glucose reacts with hydroxylamine to form an oxime.
(3) Pentaacetate of glucose does not react with hydroxylamine.
(4) Glucose is oxidised by nitric acid to gluconic acid.
Ans. (3)
Solution.
The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free -CHO group. This property of glucose can be explained only by its cyclic structure.
Q.17. Optical rotations of some compounds along with their structures are given below which of them have D configuration.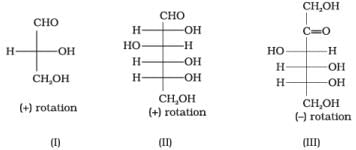
(1) I, II, III
(2) II, III
(3) I, II
(4) III
Ans. (1)
Solution.
I, II and III structures have D configuration with -OH group on the lowest asymmetric carbon is on the right side which is comparable to (+) glyceraldehyde.
Q.18. Structure of a disaccharide formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.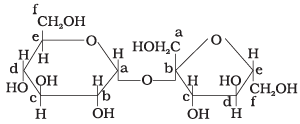 (1) ‘a’ carbon of glucose and ‘ a’ carbon of fructose.
(1) ‘a’ carbon of glucose and ‘ a’ carbon of fructose.
(2) ‘a’ carbon of glucose and ‘e’ carbon of fructose.
(3) ‘a’ carbon of glucose and ‘ b’ carbon of fructose.
(4) ‘f ’ carbon of glucose and ‘f ’ carbon of fructose.
Ans. (3)
Solution.
Carbon adjacent to oxygen atom in the cyclic structure of glucose or fructose is known as anomeric carbon. As shown in the structure above ‘a’ and ‘b’ are present at adjacent to oxygen atom. Both carbons differ in configurations of the hydroxyl group.
Q.19. Three structures are given below in which two glucose units are linked. Which of these linkages between glucose units are between C1 and C4 and which linkages are between C1 and C6?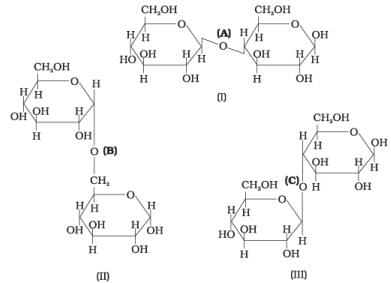 (1) (A) is between C1 and C4, (B) and (C) are between C1 and C6
(1) (A) is between C1 and C4, (B) and (C) are between C1 and C6
(2) (A) and (B) are between C1 and C4, (C) is between C1 and C6
(3) (A) and (C) are between C1 and C4, (B) is between C1 and C6
(4) (A) and (C) are between C1 and C6, (B) is between C1 and C4
Ans. (3)
Solution.
(A) and (C) are between C1 and C4, (B) is between C1 and C6.
Multiple Choice Questions -II
Note : In the following questions two or more options may be correct.
Q.20. Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non-reducing sugar. Sucrose is a ________.
(1) Monosaccharide
(2) Disaccharide
(3) Reducing sugar
(4) Non-reducing sugar
Ans. (2, 4)
Solution.
Sucrose is a disaccharide and a non-reducing sugar.
Q.21. Proteins can be classified into two types on the basis of their molecular shape i.e., fibrous proteins and globular proteins. Examples of globular proteins are :
(1) Insulin
(2) Keratin
(3) Albumin
(4) Myosin
Ans. (1, 3)
Solution.
The structure of protein which results when the chain of polypeptides coil around to give a spherical shape are known as globular protein. These proteins are soluble in water, e.g., insulin and albumin are globular protein. Hence, (a) and (c) are correct choices.
Q.22. Which of the following carbohydrates are branched polymer of glucose?
(1) Amylose
(2) Amylopectin
(3) Cellulose
(4) Glycogen
Ans. (2, 4)
Solution.
Amylopectin and glycogen are branched polymer of glucose.
Q.23. Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. Which of the following are acidic?
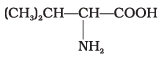
(1) 
(2)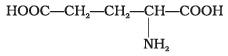
(3)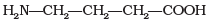
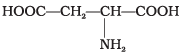
(4) 
Ans. (2, 4)
Solution.
Amino acids with more than one -COOH group one against – NH2 group are acidic in nature.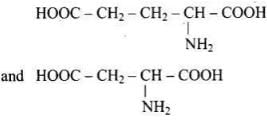
are acidic amino acids.
Q.24. Lysine,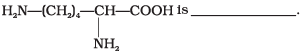
(1) α-Amino acid
(2) Basic amino acid
(3) Amino acid synthesised in body
(4) β-Amino acid
Ans. (1, 2, 3)
Solution.
Lysine whose structural formula is written as
(a) It is an a amino acid.
(b) It is a basic amino acid because number of NH2 groups (2) is greater than number of COOH group (1).
(c) It is a non-essential amino acid. Because it is synthesised in our body.
Q.25. Which of the following monosaccharides are present as five membered cyclic structure (furanose structure)?
(1) Ribose
(2) Glucose
(3) Fructose
(4) Galactose
Ans. (1, 3)
Solution.
Ribose and fructose are presented as five membered cyclic structure (furanose structures). They have five membered ring with analogy to the compound foran.
Q.26. In fibrous proteins, polypeptide chains are held together by ___________.
(1) Van der Waals forces
(2) Disulphide linkage
(3) Electrostatic forces of attraction
(4) Hydrogen bonds
Ans. (2, 4)
Solution.
In fibrous proteins, polypeptide chains are held together by disulphide linkage and hydrogen bonds.
Q.27. Which of the following are purine bases?
(1) Guanine
(2) Adenine
(3) Thymine
(4) Uracil
Ans. (1, 2)
Solution.
Purines consist of six membered and five membered nitrogen containing ring fused together.
Guanine and adenine are purine bases whose structures are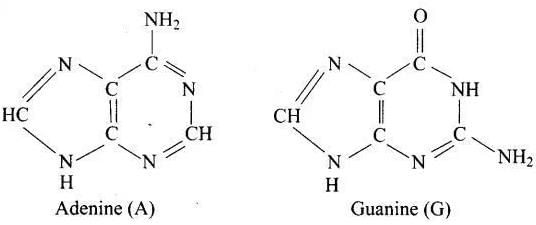
While thymine and uracil are pyrimidine bases.
Hence (a) and (b) are correct choices.
Q.28. Which of the following terms are correct about enzyme?
(1) Proteins
(2) Dinucleotides
(3) Nucleic acids
(4) Biocatalysts
Ans. (1, 4)
Solution.
Enzymes are protein molecules and they act as biocatalysts for the reactions taking place in the body.
Short Answer Type Questions
Q.29. Name the sugar present in milk. How many monosaccharide units are present in it? What are such oligosaccharides called?
Ans. Lactose is present in milk. Two monosaccharide units (i.e., glucose and galactose) are present in it. Such oligosaccharides are called disaccharides.
Q.30. How do you explain the presence of all the six carbon atoms in glucose in a straight chain?
Ans. Glucose on prolonged heating with HI and red phosphorus gives n-hexane HI (excess)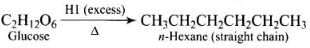
Q.31. In nucleoside a base is attached at 1′ position of sugar moiety. Nucleotide is formed by linking of phosphoric acid unit to the sugar unit of nucleoside. At which position of sugar unit is the phosphoric acid linked in a nucleoside to give a nucleotide?
Ans. Phosphoric acid unit is linked preferably at 5′-position of sugar moiety of a nucleoside to give a nucleotide.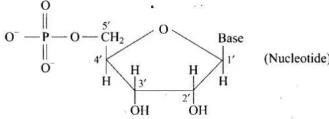
Q.32. Name the linkage connecting monosaccharide units in polysaccharides.
Ans. The monosaccharide units are linked through glycosidic linkage in a polysaccharide.
Q.33. Under what conditions glucose is converted to gluconic and saccharic acid?
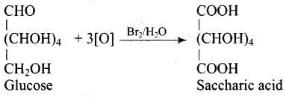
Ans. Glucose is converted to gluconic acid by Br2 water and to saccharic acid by conc. HNO3.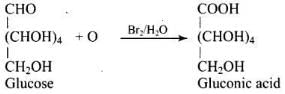
Q.34. Monosaccharides contain carbonyl group hence are classified, as aldose or ketose. The number of carbon atoms present in the monosaccharide molecule are also considered for classification. In which class of monosaccharide will you place fructose?
Ans. Monosaccharides contain carbonyl group. Hence, are classified as aldose or ketose. When aldehyde group is present, the monosaccharides are known as aldose. When ketone group is present, the monosaccharides are known as ketose. Fructose has molecular formula C6H12O6 containing six carbon and keto group and is classified as ketohexose.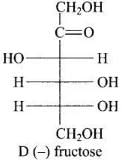
Q.35. The letters ‘D’ or ‘L’ before the name of a stereoisomer of a compound indicate the correlation of configuration of that particular stereoisomer. This refers to their relation with one of the isomers of glyceraldehyde. Predict whether the following compound has ‘D’ or ‘L’ configuration.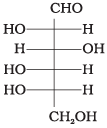 Ans. Since the -OH group at the penultimate chiral carbon atom (i.e., last but one or C5) is towards left. Therefore, the given compound has L-configuration.
Ans. Since the -OH group at the penultimate chiral carbon atom (i.e., last but one or C5) is towards left. Therefore, the given compound has L-configuration.
Q.36. Aldopentoses named as ribose and 2-deoxyribose are found in nucleic acids. What is their relative configuration?
Ans. Both the aldopentoses have D-configuration.
Q.37. Which sugar is called invert sugar? Why is it called so?
Ans. Sucrose is called invert sugar. The sugar obtained from sugar beet is a colourless, crystalline and sweet substance. It is very soluble in water and its aqueous solution is dextrorotatory having [α]D = + 66.5°. On hydrolysis with dilute acids or enzyme invertase, cane sugar gives equimolar mixture of D-(+)-glucose and D-(-)-fructose.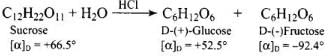 So, sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose. D-(+)-fructose has a greater specific rotation than D-(+)-glucose. Therefore, the resultant solution upon hydrolysis is laevorotatory in nature with specific rotation of (-39.9°). Since there is change in the sign of rotation from dextro before hydrolysis to laevo after hydrolysis, the reaction is called inversion reaction and the mixture (glucose and fructose) is called invert sugar.
So, sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose. D-(+)-fructose has a greater specific rotation than D-(+)-glucose. Therefore, the resultant solution upon hydrolysis is laevorotatory in nature with specific rotation of (-39.9°). Since there is change in the sign of rotation from dextro before hydrolysis to laevo after hydrolysis, the reaction is called inversion reaction and the mixture (glucose and fructose) is called invert sugar.
Q.38. Amino acids can be classified as α-, β-, γ-, δ- and so on depending upon the relative position of amino group with respect to carboxyl group. Which type of amino acids form polypetide chain in proteins?
Ans. α - Amino acid,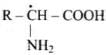 forms polypeptide chain in proteins.
forms polypeptide chain in proteins.
Q.39. α-Helix is a secondary structure of proteins formed by twisting of polypeptide chain into right handed screw like structures. Which type of interactions are responsible for making the α-helix structure stable?
Ans. In a-helix structure of protein, a polypeptide chain is stabilize by the formation of intramolecular H-bonding between -NH- group of amino acids in one turn with the >C = O groups of amino acids belonging to adjacent turn.
Q.40. Some enzymes are named after the reaction, where they are used. What name is given to the class of enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate.
Ans. Enzyme oxidoreductase.
Q.41. During curdling of milk, what happens to sugar present in it?
Ans. The milk sugar lactose is converted into lactic acid during curdling of milk.
Q.42. How do you explain the presence of five —OH groups in glucose molecule?
Ans. Glucose gives penta-acetyl derivative on acetylation with acetic anhydride.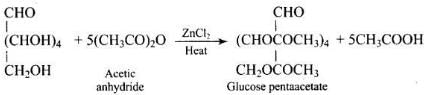
This confirms the presence of five -OH groups.
Q.43. Why does compound (A) given below not form an oxime?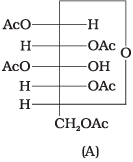 Ans. Glucose pentaacetate (structure A) does not have a free -OH group at C, and therefore, cannot be converted to the open chain form to give a free -CHO group and hence it does not form the oxime.
Ans. Glucose pentaacetate (structure A) does not have a free -OH group at C, and therefore, cannot be converted to the open chain form to give a free -CHO group and hence it does not form the oxime.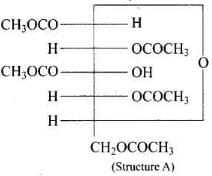
Q.44. Why must vitamin C be supplied regularly in diet?
Ans. Vitamin ‘C’ is water soluble and hence excess of it is readily excreted in urine so, it cannot be stored in our body and hence, it should be regularly supplied in diet.
Q.45. Sucrose is dextrorotatory but the mixture obtained after hydrolysis is laevorotatory. Explain.
Ans. Sucrose is dextrorotatory having [α]D = + 66.5° On hydrolysis with dilute acids or enzymes, it gives equimolar D-(+)-glucose and D-(-)fructose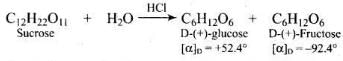
Since D-(-)-fructose has larger specific rotation than D-(-)-fructose has larger specific rotation than D-(+)-glucose, the resulting mixture has specific rotation of -39.9°. Therefore, the mixture is laevorotatory.
Q.46. Amino acids behave like salts rather than simple amines or carboxylic acids.Explain.
Ans. In aqueous solution, the -COOH group of an amino acid loses a proton and -NH2 group accepts a proton to form zwitter ion (salt).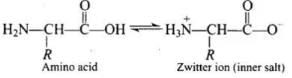
Q.47. Structures of glycine and alanine are given below. Show the peptide linkage in glycylalanine.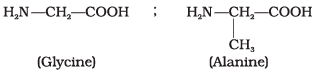 Ans. The carboxyl group of glycine and amino group of alanine when combines together, they form glycylalanine through a peptide (-CO – NH) linkage.
Ans. The carboxyl group of glycine and amino group of alanine when combines together, they form glycylalanine through a peptide (-CO – NH) linkage.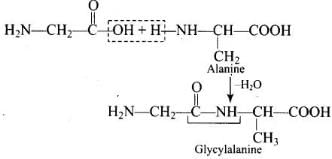
Q.48. Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein. When a protein in its native form, is subjected to a physical change like change in temperature or a chemical change like, change in pH, denaturation of protein takes place. Explain the cause.
Ans. Due to physical or chemical change, hydrogen bonding and various other attractive forces are disturbed, globules unfold and helix gets uncoiled to form a thread like molecule. Therefore, secondary and tertiary structure of protein loses all or part of their biological activity. This is called denaturation of proteins.
Q.49. Activation energy for the acid catalysed hydrolysis of sucrose is 6.22 kJ mol–1, while the activation energy is only 2.15 kJ mol–1 when hydrolysis is catalysed by the enzyme sucrase. Explain.
Ans. Enzymes are biocatalysts. They reduce the magnitude of activation energy by providing alternative path. In the hydrolysis of sucrose, the enzyme sucrase reduces the activation energy from 6.22 kJ mol-1 to 2.15 kJ mol-1. As a result, enzyme catalysed reactions occur at a much faster rate than the ordinary chemicdl reactions using conventional catalysts.
Q.50. How do you explain the presence of an aldehydic group in a glucose molecule?
Ans. Glucose reacts with hydroxylamine to form a monoxime, and adds one molecule of hydrogen cyanide to give cyanohydrin.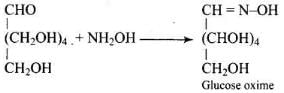
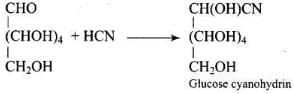
Therefore, it contains a carbonyl group which can be an aldehyde or a ketone. On mild oxidation with bromine water, glucose gives gluconic acid which is a carboxylic acid containing six carbon atoms.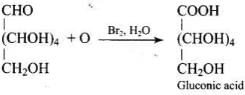
This indicates that carbonyl group present in glucose is an aldehydic group.
Q.51. Which moieties of nucleosides are involved in the formation of phosphodiester linkages present in dinucleotides? What does the word diester in the name of linkage indicate? Which acid is involved in the formation of this linkage?
Ans. Nucleosides are linked to phosphoric acid at 5′-position of sugar moiety to form a nucleotide. Further, nucleotides (two molecules) are joined together by phosphodiester linkage between 5′ and 3′ carbon atoms of pentose sugar to form dinucleotide. Phosphoric acid is involved in the formation of this linkage.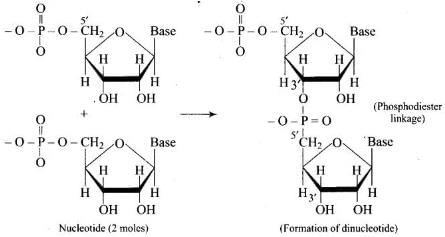
Q.52. What are glycosidic linkages? In which type of biomolecules are they present?
Ans. Two molecules of monosaccharides are joined together by an oxide linkage formed by the loss of water molecule. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Glycosidic linkage is present in disaccharides, trisaccharides and polysaccharides, etc.
Q.53. Which monosaccharide units are present in starch, cellulose and glucose and which linkages link these units?
Ans. In starch a-glucose units are present, in cellulose β-D glucose units are present. In starch and glycogen glycosidic a-linkage is present and in cellulose glycosidic β-linkage is present between glucose units.
Q.54. How do enzymes help a substrate to be attacked by the reagent effectively?
Ans. Active site of enzymes hold the substrate molecule in a suitable position, so that it can be attacked by the reagent effectively.
Q.55. Describe the term D- and L- configuration used for amino acids with examples.
Ans. The sugars are divided into two families: the D-family and L-family which have definite configurations. These configurations are represented with respect to glyceraldehyde as the standard. The glyceraldehydes may be presented by two forms as:
D-(+)-Glyceraldehyde L-(-)-Glyceraldehyde The D-configuration has -OH attached to the carbon adjacent to -CH2OH on right while L-configuration has -OH attached to the carbon adjacent to -CH2OH on left. The sugars are called D- or L-depending upon whether the configuration of the molecule is related to D-glyceraldehyde or L-glyceraldehyde. It has been found that all naturally occurring sugars belong to D-series, e.g., D-glucose D-ribose and D-fructose.
Q.56. How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
Ans. Glucose on treatment with acetic anhydride in presence of pyridine or a few drops of cone. H2SO4, it forms penta-acetyl derivative indicating the presence of 5 -OH groups. Out of which one -OH group is primary (1°) alcoholic and four (C2, C3, C4 and C5) -OH group are secondary (2°) alcoholic groups.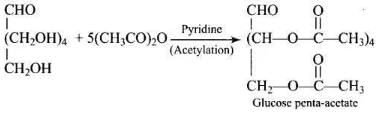
Glucose (or gluconic acid) on oxidation with HNO3 gives saccharic acid (a dicarboxylic acid) indicating that one of the primary (1°) alcoholic group is oxidized to -COOH group but (2°) hydroxyl groups undergo oxidation only under drastic conditions.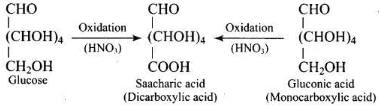
Q.57. Coagulation of egg white on boiling is an example of denaturation of protein. Explain it in terms of structural changes.
Ans. When an egg is boiled, the soluble globular protein, albumin present in it is converted into insoluble fibrous protein. During this denaturation
(i) biological activity is lost and
(ii) secondary and tertiary structures of albumin protein are destroyed while the primary structure (representing the sequence of α-amino acids) remains intact.
Matching Type Questions
Note : Match the items of Column I and Column II in the following questions.
More than one option in Column II may match with the items given in Column I.
Q.58. Match the vitamins given in Column I with the deficiency disease they cause given in Column II.
| Column I (Vitamins) | Column II (Diseases) |
| (i) Vitamin A | (a) Pernicious anaemia |
| (ii) Vitamin B1 | (b) Increased blood clotting time |
| (iii) Vitamin B12 | (c) Xerophthalmia |
| (iv) Vitamin C | (d) Rickets |
| (v) Vitamin D | (e) Muscular weakness |
| (vi) Vitamin E | (f) Night blindness |
| (vii) Vitamin K | (g) Beri Beri |
| (h) Bleeding gums | |
| (i) Osteomalacia |
Ans. (i → c, f), (ii → g), (iii → a), (iv → h), (v → d,i), (vi → e), (vii → b)
Q.59. Match the following enzyms given in Column I with the reactions they catalyse given in Column II.
| Column I (Enzymes) | Column II (Reactions) |
| (i) Invertase | (a) Decomposition of urea into NH3 and CO2 |
| (ii) Maltase | (b) Conversion of glucose into ethyl alcohol |
| (iii) Pepsin | (c) Hydrolysis of maltose into glucose |
| (iv) Urease | (d) Hydrolysis of cane sugar |
| (v) Zymase | (e) Hydrolysis of proteins into peptides |
Ans. (i → d), (ii → c), (iii → e), (iv → a), (v → b)
ASSERTION AND REASON TYPE QUESTIONS
Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Q.60. Assertion : D (+) – Glucose is dextrorotatory in nature.
Reason : ‘D’ represents its dextrorotatory nature.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (c)
Solution.
‘D’ corresponds to the position of -OH group on the right side on the farthest asymmetric C-atom.
Q.61. Assertion : Vitamin D can be stored in our body.
Reason : Vitamin D is fat soluble vitamin.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (a)
Solution.
Vitamin D can be stored in our body because it is a fat soluble vitamin.
Q.62. Assertion : β-glycosidic linkage is present in maltose,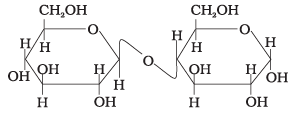 Reason : Maltose is composed of two glucose units in which C–1 of one glucose unit is linked to C–4 of another glucose unit.
Reason : Maltose is composed of two glucose units in which C–1 of one glucose unit is linked to C–4 of another glucose unit.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (d)
Solution.
α-glycosidic linkage is present in maltose.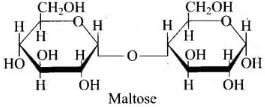
Q.63. Assertion : All naturally occurring α-aminoacids except glycine are optically active.
Reason : Most naturally occurring amino acids have L-configuration.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (e)
Solution.
All α-amino acids except glycine contain at least one chiral carbon.
Q.64. Assertion : Deoxyribose, C5H10O4 is not a carbohydrate.
Reason : Carbohydrates are hydrates of carbon so compounds which follow Cx(H2O)y formula are carbohydrates.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (b)
Solution.
Deoxyribose is a carbohydrate and is the sugar moiety of DNA. Carbohydrates are optically active polyhydroxy aldehyde or polyhydroxy ketone or substances which give these on hydrolysis.
Q.65. Assertion : Glycine must be taken through diet.
Reason : It is an essential amino acid.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (b)
Solution.
Glycine can be synthesized by the body and is a non-essential amino acid.
Q.66. Assertion : In presence of enzyme, substrate molecule can be attacked by the reagent effectively.
Reason : Active sites of enzymes hold the substrate molecule in a suitable position.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans. (a)
Solution.
In presence of enzyme substrate molecule can be attacked by the reagent effectively because active sites of enzymes hold the substrate molecule in a suitable position.
Long Answer Type Questions
Q.67. Write the reactions of D-glucose which can’t be explained by its open-chain structure. How can cyclic structure of glucose explain these reactions?
Ans. The open chain structure of glucose explained most of its properties. However, it could not explain the following facts.
(i) Despite having an aldehydic (-CHO) group, glucose does not undergo certain characteristic Reactions of aldehydes. For example,
(a) Glucose does not react with ammonia.
(b) Glucose does not react with sodium bisulphite (NaHSO3) to form addition product.
(c) Glucose does not give Schiff’s test and 2, 4-DNP test like other aldehydes.
(ii) Glucose reacts with hydroxylamine (-NH2OH) to form an oxime but glucose pentaacetate does not react with hydroxylamine. This shows that -CHO groups is not present in glucose pentaacetate.
(iii) D (+) – Glucose exists in two stereoisomeric forms i.e., α -D-glucose and β-D-glucose. These two forms are crystalline and have different melting points and optical rotations.
(iv) An aqueous solution of glucose shows mutarotation, i.e., its specific rotation gradually decreases from +110° to + 52.5° in case of α -glucose and increases from +19.7° to + 52.5° in case of β-glucose.
(v) Glucose forms isomeric methyl glucosides. When glucose is heated with methanol in the presence of dry hydrogen chloride gas, it gives two isomeric monomethyl derivatives known as methyl α -D-glucoside (m.p. = 438 K or 165°C) and methyl β-D-glucoside (m.p. = 380 K or 107°C).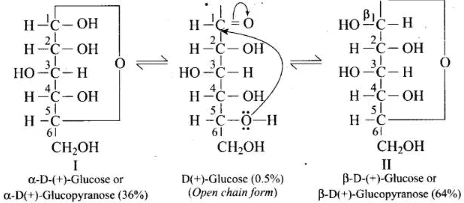 These results shows that glucose does not have open chain form structure.
These results shows that glucose does not have open chain form structure.
Q.68. On the basis of which evidences D-glucose was assigned the following structure?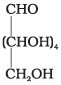 Ans. This structure was assigned on the basis of the following evidences:
Ans. This structure was assigned on the basis of the following evidences:
1. Molecular formula: The molecular formula of glucose has been found to be C6H12O6.
2. Straight chain structure: (i) When aqueous solution of glucose is treated with sodium amalgam (Na/Hg) or sodium borohydride. it is reduced to sorbitol (or glucitol) a hexahydric alcohol. (ii) Prolonged heating with hydriodic acid and red phosphorus at 100°C gives a mixture of n-hexane and 2-iodohexane.
(ii) Prolonged heating with hydriodic acid and red phosphorus at 100°C gives a mixture of n-hexane and 2-iodohexane.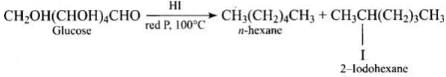 The formation of n-hexane suggests that all the six carbon atoms in glucose are arranged in a straight chain structure of glucose.
The formation of n-hexane suggests that all the six carbon atoms in glucose are arranged in a straight chain structure of glucose.
3. Presence of five hydroxyl (-OH) groups: On acetylation with acetic anhydride, glucose gives a pentaacetate. This confirms that glucose contains five -OH groups. We know that the presence of two or more -OH groups on the same carbon atom makes the molecules unstable.
Now since glucose is a stable compound, therefore, the five -OH groups must present on different carbon atoms.
4. Presence of one primary alcoholic group: On oxidation with cone, nitric acid, both glucose and gluconic acid give the same dicarboxylic acid, saccharic acid or glucaric acid. The primary alcoholic group (CH2OH) is always present at the end of the carbon chain.
5. Presence of an aldehyde (-CHO) group: Glucose reacts with hydroxylamine, NH2OH to form glucose CHO oxime. Which suggest that glucose contains a carbonyl (CHOH)4 (>C = O) groups.
On the basis of above observations, the following open- CH2OH chain structure for glucose can be written as follows: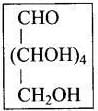
Q.69. Carbohydrates are essential for life in both plants and animals. Name the carbohydrates that are used as storage molecules in plants and animals, also name the carbohydrate which is present in wood or in the fibre of cotton cloth.
Ans. Carbohydrate that are used as storage molecules in plants and animals are as follows
(i) Plant contains mainly starch, cellulose, sucrose, etc.
(ii) Animal contain glycogen in their body. So, glycogen is also known as animal starch. Glycogen is present in liver, muscles and brain when body needs glucose, enzyme breaks glycogen down to glucose.
(iii) Cellulose is present in wood, and fibre of clothes.
Q.70. Explain the terms primary and secondary structure of proteins. What is the difference between α-helix and β-pleated sheet structure of proteins?
Ans. Primary structure: Proteins may have one or more polypeptide chains. Each polypeptide chain has a large number of a-amino acids linked to one another in a specific sequence. The specific sequence in which the various α-amino acids present in a protein are linked to one another is called its primary structure. Any change in this primary structure, i.e., sequence of amino acids creates a different protein.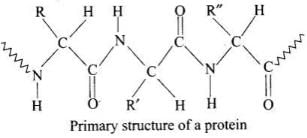 The primary structure of a protein is usually determined by its successive hydrolysis with either enzymes or mineral acids into various products having decreasing molecular mass as shown below:
The primary structure of a protein is usually determined by its successive hydrolysis with either enzymes or mineral acids into various products having decreasing molecular mass as shown below:
Secondary structure: The secondary structure gives the manner in which the polypeptide chains are folded or arranged. Therefore, it gives the shape or conformation of the protein molecule.
This arises from the plane geometry, of the peptide bond and hydrogen bond between the >C = O and N – H groups of different peptide bonds.
α-Helix structure: a-helix form is the most common form in which a polypeptide chain forms all possible types of hydrogen bonds by twisting into a right handed screw (helix) with the -NH group of each amino acid residue hydrogen bonded to -C = O group of the adjacent turn of the helix. The a-helix structure is also known as 3.613 helix. This represents that each turn of the helix contains approximately 3.6 amino acids and a 13-member ring is formed by hydrogen bonding. The helix is held in its shape primarily by hydrogen bonds between one amide group and carbonyl group which is 3.6 amino acids units away.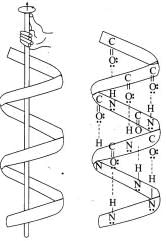
The α-Helix structure of proteins
β-pleated sheet structure: In this conformation, the polypeptide chains lie side by side in a zigzag manner with alternate R groups on the same side situated at fixed distances apart. The two such neighbouring polypeptide chains are held together by intermolecular H-bonds. A number of such chains can be interbounded to form a sheet. These sheets are then stacked one above the other like the pages of this book to form a three-dimensional structure. This structure resembles pleated folds of drapery and hence is also called β-pleated sheet structure.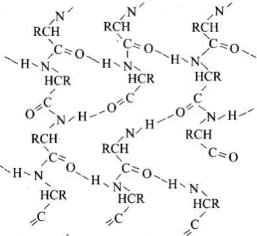
β-Pleated sheet structure of proteins
Q.71. Write the structures of fragments produced on complete hydrolysis of DNA. How are they linked in DNA molecule? Draw a diagram to show pairing of nucleotide bases in double helix of DNA.
Ans. Complete hydrolysis of DNA yields a pentose sugar, phosphoric acid and nitrogen containing heterocyclic compounds called bases.
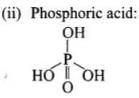
Structures: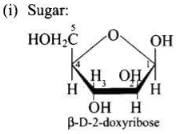
 (iii) Nitrogen base: DNA contains four base Adenine (A), Guanine (G), Cytosine (C) and Thymine (T).
(iii) Nitrogen base: DNA contains four base Adenine (A), Guanine (G), Cytosine (C) and Thymine (T).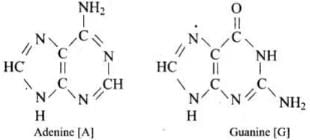
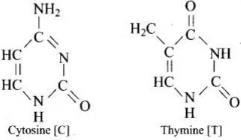 A unit formed by the attachment of a base to I ‘-position of sugar is called nucleoside. When nucleoside links to phosphoric acid at 5′-position of sugar moiety, a nucleotide is formed. Nucleotides are joined together by phosphodiester linkage between 5’-and 3’carbon atoms of the pentose sugar.
A unit formed by the attachment of a base to I ‘-position of sugar is called nucleoside. When nucleoside links to phosphoric acid at 5′-position of sugar moiety, a nucleotide is formed. Nucleotides are joined together by phosphodiester linkage between 5’-and 3’carbon atoms of the pentose sugar.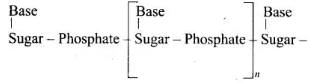 In DNA, two chains of nucleic acid coil about each other and held together by H-bonds between bases of two chains.
In DNA, two chains of nucleic acid coil about each other and held together by H-bonds between bases of two chains.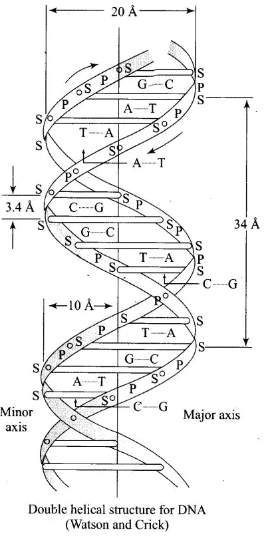
|
75 videos|278 docs|78 tests
|
FAQs on NCERT Exemplar: Biomolecules - Chemistry Class 12 - NEET
| 1. What are the main types of biomolecules? |  |
| 2. How do enzymes function as biological catalysts? |  |
| 3. What is the significance of DNA and RNA in living organisms? |  |
| 4. What are the functions of carbohydrates in biological systems? |  |
| 5. How do lipids contribute to cellular structure and function? |  |





















