NCERT QUESTION
(Nuclei)
Q13.1: Obtain the binding energy (in MeV) of a nitrogen nucleus 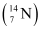 , given
, given 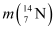 =14.00307 u
=14.00307 u
Ans: Atomic mass of nitrogen 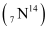 , m = 14.00307 u
, m = 14.00307 u
A nucleus of nitrogen 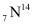 contains 7 protons and 7 neutrons.
contains 7 protons and 7 neutrons.
Hence, the mass defect of this nucleus, Δm = 7mH 7mn − m
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn= 1.008665 u
∴Δm = 7 × 1.007825 7 × 1.008665 − 14.00307
= 7.054775 + 7.06055 − 14.00307
= 0.11236 u
But 1 u = 931.5 MeV/c2
∴Δm = 0.11236 × 931.5 MeV/c2
Hence, the binding energy of the nucleus is given as:
Eb = Δmc2
Where,
c = Speed of light
∴Eb = 0.11236 × 931.5 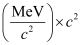
= 104.66334 MeV
Hence, the binding energy of a nitrogen nucleus is 104.66334 MeV.
Q13.2: Obtain the binding energy of the nuclei 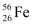 and
and 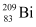 in units of MeV from the following data:
in units of MeV from the following data:
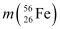 = 55.934939 u ,
= 55.934939 u , 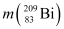 = 208.980388 u
= 208.980388 u
Ans: Atomic mass of 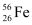 , m1 = 55.934939 u
, m1 = 55.934939 u
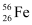 nucleus has 26 protons and (56 − 26) = 30 neutrons
nucleus has 26 protons and (56 − 26) = 30 neutrons
Hence, the mass defect of the nucleus, Δm = 26 × mH 30 × mn − m1
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn = 1.008665 u
∴Δm = 26 × 1.007825 30 × 1.008665 − 55.934939
= 26.20345 + 30.25995 − 55.934939
= 0.528461 u
But 1 u = 931.5 MeV/c2
∴Δm = 0.528461 × 931.5 MeV/c2
The binding energy of this nucleus is given as:
Eb1 = Δmc2
Where,
c = Speed of light
∴ Eb1 = 0.528461 × 931.5 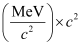
= 492.26 MeV
Average binding energy per nucleon 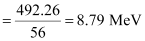
Atomic mass of 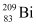 , m2 = 208.980388 u
, m2 = 208.980388 u
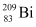 nucleus has 83 protons and (209 − 83) 126 neutrons.
nucleus has 83 protons and (209 − 83) 126 neutrons.
Hence, the mass defect of this nucleus is given as:
Δm' = 83 × mH 126 × mn − m2
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn = 1.008665 u
∴Δm' = 83 × 1.007825 126 × 1.008665 − 208.980388
= 83.649475 + 127.091790 − 208.980388
= 1.760877 u
But 1 u = 931.5 MeV/c2
∴Δm' = 1.760877 × 931.5 MeV/c2
Hence, the binding energy of this nucleus is given as:
Eb2 = Δm'c2
= 1.760877 × 931.5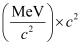
= 1640.26 MeV
Average binding energy per nucleon = 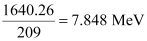
Q13.3: A given coin has a mass of 3.0 g. Calculate the nuclear energy that would be required to separate all the neutrons and protons from each other. For simplicity assume that the coin is entirely made of 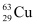 atoms (of mass 62.92960 u).
atoms (of mass 62.92960 u).
Ans: Mass of a copper coin, m’ = 3 g
Atomic mass of 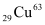 atom, m = 62.92960 u
atom, m = 62.92960 u
The total number of 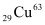 atoms in the coin
atoms in the coin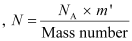
Where,
NA = Avogadro’s number = 6.023 × 1023 atoms /g
Mass number = 63 g
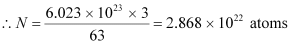
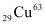 nucleus has 29 protons and (63 − 29) 34 neutrons
nucleus has 29 protons and (63 − 29) 34 neutrons
∴ Mass defect of this nucleus, Δm' = 29 × mH 34 × mn − m
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn = 1.008665 u
∴ Δm' = 29 × 1.007825 34 × 1.008665 − 62.9296
= 0.591935 u
Mass defect of all the atoms present in the coin, Δm = 0.591935 × 2.868 × 1022
= 1.69766958 × 1022 u
But 1 u = 931.5 MeV/c2
∴Δm = 1.69766958 × 1022 × 931.5 MeV/c2
Hence, the binding energy of the nuclei of the coin is given as:
Eb= Δmc2
= 1.69766958 × 1022 × 931.5 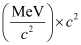
= 1.581 × 1025 MeV
But 1 MeV = 1.6 × 10−13 J
Eb = 1.581 × 1025 × 1.6 × 10−13
= 2.5296 × 1012 J
This much energy is required to separate all the neutrons and protons from the given coin.
Q13.4: Obtain approximately the ratio of the nuclear radii of the gold isotope 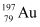 and the silver isotope
and the silver isotope 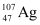 .
.
Ans: Nuclear radius of the gold isotope 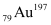 = RAu
= RAu
Nuclear radius of the silver isotope 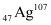 = RAg
= RAg
Mass number of gold, AAu = 197
Mass number of silver, AAg = 107
The ratio of the radii of the two nuclei is related with their mass numbers as:
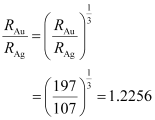
Hence, the ratio of the nuclear radii of the gold and silver isotopes is about 1.23.
Q13.5: The Q value of a nuclear reaction A + b → C + d is defined by Q = [ mA + mb− mC− md]c2 where the masses refer to the respective nuclei. Determine from the given data the Q-value of the following reactions and state whether the reactions are exothermic or endothermic.
(i) 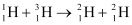
(ii) 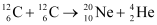
Atomic masses are given to be
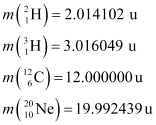
Ans: (i) The given nuclear reaction is:
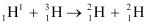
It is given that:
Atomic mass 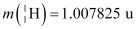
Atomic mass 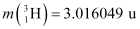
Atomic mass 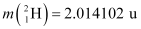
According to the question, the Q-value of the reaction can be written as:
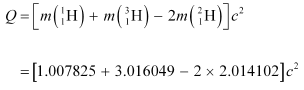
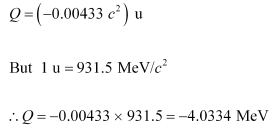
The negative Q-value of the reaction shows that the reaction is endothermic.
(ii) The given nuclear reaction is:
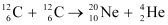
It is given that:
Atomic mass of 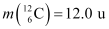
Atomic mass of 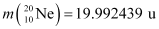
Atomic mass of 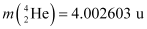
The Q-value of this reaction is given as:
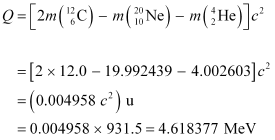
The positive Q-value of the reaction shows that the reaction is exothermic.
Q13.6: Suppose, we think of fission of a 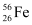 nucleus into two equal fragments,
nucleus into two equal fragments, 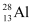 . Is the fission energetically possible? Argue by working out Q of the process. Given
. Is the fission energetically possible? Argue by working out Q of the process. Given 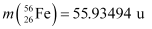 and
and 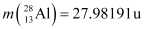 .
.
Ans: The fission of 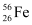 can be given as:
can be given as:
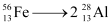
It is given that:
Atomic mass of 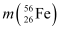 = 55.93494 u
= 55.93494 u
Atomic mass of 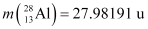
The Q-value of this nuclear reaction is given as:
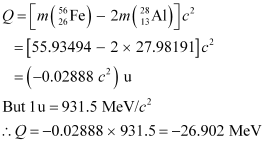
The Q-value of the fission is negative. Therefore, the fission is not possible energetically. For an energetically-possible fission reaction, the Q-value must be positive.
Q13.7: The fission properties of 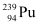 are very similar to those of
are very similar to those of 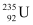 . The average energy released per fission is 180 MeV. How much energy, in MeV, is released if all the atoms in 1 kg of pure
. The average energy released per fission is 180 MeV. How much energy, in MeV, is released if all the atoms in 1 kg of pure 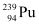 undergo fission?
undergo fission?
Ans: Average energy released per fission of 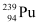 ,
, 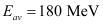
Amount of pure 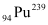 , m = 1 kg = 1000 g
, m = 1 kg = 1000 g
NA= Avogadro number = 6.023 × 1023
Mass number of 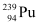 = 239 g
= 239 g
1 mole of 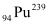 contains NA atoms.
contains NA atoms.
∴ m g of 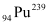 contains
contains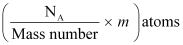
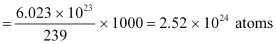
∴Total energy released during the fission of 1 kg of 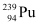 is calculated as:-
is calculated as:-
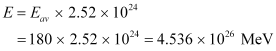
Hence, 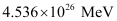 is released if all the atoms in 1 kg of pure
is released if all the atoms in 1 kg of pure 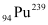 undergo fission.
undergo fission.
Q13.8: How long can an electric lamp of 100W be kept glowing by fusion of 2.0 kg of deuterium? Take the fusion reaction as
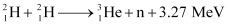
Ans: The given fusion reaction is:
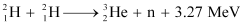
Amount of deuterium, m = 2 kg
1 mole, i.e., 2 g of deuterium contains 6.023 × 1023 atoms.
∴2.0 kg of deuterium contains 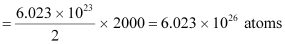
It can be inferred from the given reaction that when two atoms of deuterium fuse, 3.27 MeV energy is released.
∴ Total energy per nucleus released in the fusion reaction:
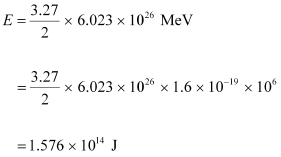
Power of the electric lamp, P = 100 W = 100 J/s.
Hence, the energy consumed by the lamp per second = 100 J
The total time for which the electric lamp will glow is calculated as:
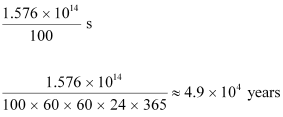
Q13.9: Calculate the height of the potential barrier for a head on collision of two deuterons. (Hint: The height of the potential barrier is given by the Coulomb repulsion between the two deuterons when they just touch each other. Assume that they can be taken as hard spheres of radius 2.0 fm.)
Ans: When two deuterons collide head-on, the distance between their centres, d is given as:
Radius of 1st deuteron Radius of 2nd deuteron
Radius of a deuteron nucleus = 2 fm = 2 × 10−15 m
∴ d = 2 × 10−15 2 × 10−15 = 4 × 10−15 m
Charge on a deuteron nucleus = Charge on an electron = e = 1.6 × 10−19 C
Potential energy of the two-deuteron system:
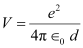
Where,
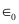 = Permittivity of free space
= Permittivity of free space
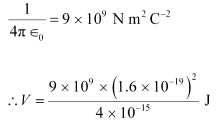
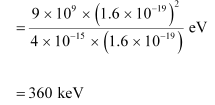
Hence, the height of the potential barrier of the two-deuteron system is 360 keV.
Q13.10: From the relation R = R0A1/3, where R0 is a constant and A is the mass number of a nucleus, show that the nuclear matter density is nearly constant (i.e. independent of A).
Ans: We have the expression for nuclear radius as:
R = R0A1/3
Where,
R0 = Constant.
A = Mass number of the nucleus
Nuclear matter density, 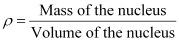
Let m be the average mass of the nucleus.
Hence, mass of the nucleus = mA
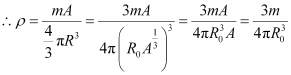
Hence, the nuclear matter density is independent of A. It is nearly constant.
Old NCERT Solutions
Q1: For the 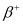 (positron) emission from a nucleus, there is another competing process known as electron capture (electron from an inner orbit, say, the K−shell, is captured by the nucleus and a neutrino is emitted).
(positron) emission from a nucleus, there is another competing process known as electron capture (electron from an inner orbit, say, the K−shell, is captured by the nucleus and a neutrino is emitted).
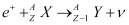
Show that if 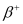 emission is energetically allowed, electron capture is necessarily allowed but not vice−versa.
emission is energetically allowed, electron capture is necessarily allowed but not vice−versa.
Ans: Let the amount of energy released during the electron capture process be Q1. The nuclear reaction can be written as:

Let the amount of energy released during the positron capture process be Q2. The nuclear reaction can be written as:

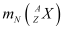 = Nuclear mass of
= Nuclear mass of 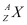
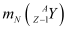 = Nuclear mass of
= Nuclear mass of 
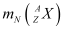 = Atomic mass of
= Atomic mass of 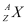
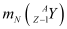 = Atomic mass of
= Atomic mass of 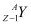
me = Mass of an electron
c = Speed of light
Q-value of the electron capture reaction is given as:
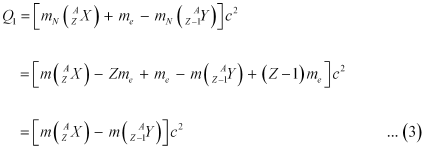
Q-value of the positron capture reaction is given as:
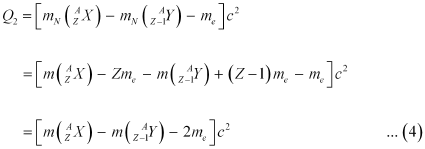
It can be inferred that if Q2 > 0, then Q1 > 0; Also, if Q1> 0, it does not necessarily mean that Q2 > 0.
In other words, this means that if 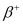 emission is energetically allowed, then the electron capture process is necessarily allowed, but not vice-versa. This is because the Q-value must be positive for an energetically-allowed nuclear reaction.
emission is energetically allowed, then the electron capture process is necessarily allowed, but not vice-versa. This is because the Q-value must be positive for an energetically-allowed nuclear reaction.
Q2: In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are 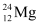 (23.98504u),
(23.98504u), 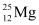 (24.98584u) and
(24.98584u) and 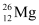 (25.98259u). The natural abundance of
(25.98259u). The natural abundance of 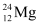 is 78.99% by mass. Calculate the abundances of other two isotopes.
is 78.99% by mass. Calculate the abundances of other two isotopes.
Ans: Average atomic mass of magnesium, m = 24.312 u
Mass of magnesium isotope 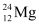 , m1 = 23.98504 u
, m1 = 23.98504 u
Mass of magnesium isotope 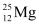 , m2 = 24.98584 u
, m2 = 24.98584 u
Mass of magnesium isotope 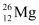 , m3 = 25.98259 u
, m3 = 25.98259 u
Abundance of 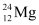 , η1= 78.99%
, η1= 78.99%
Abundance of 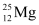 , η2 = x%
, η2 = x%
Hence, abundance of 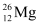 , η3 = 100 − x − 78.99% = (21.01 − x)%
, η3 = 100 − x − 78.99% = (21.01 − x)%
We have the relation for the average atomic mass as:
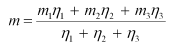
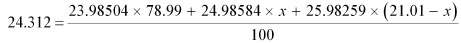
2431,2 = 1894.5783096 + 24.98584 x + 545.8942159 - 25.98259 x
0.99675* = 9.2725255
∴ x ≈ 9.3%
And 21.01 -* = 11.71%
Hence, the abundance of 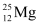 is 9.3% and that of
is 9.3% and that of 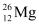 is 11.71%.
is 11.71%.
Q3: The neutron separation energy is defined as the energy required to remove a neutron from the nucleus. Obtain the neutron separation energies of the nuclei 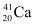 and
and 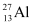 from the following data:
from the following data:
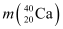 = 39.962591 u
= 39.962591 u
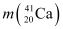 = 40.962278 u
= 40.962278 u
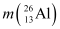 = 25.986895 u
= 25.986895 u
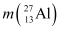 = 26.981541 u
= 26.981541 u
Ans: For 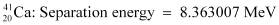
For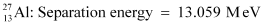
A neutron 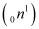 is removed from a
is removed from a 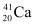 nucleus. The corresponding nuclear reaction can be written as:
nucleus. The corresponding nuclear reaction can be written as:
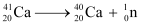
It is given that:
Mass 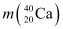 = 39.962591 u
= 39.962591 u
Mass 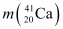 = 40.962278 u
= 40.962278 u
Mass 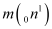 = 1.008665 u
= 1.008665 u
The mass defect of this reaction is given as:
Δm = 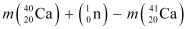
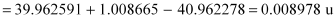
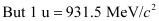
∴Δm = 0.008978 × 931.5 MeV/c2
Hence, the energy required for neutron removal is calculated as:
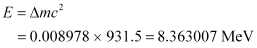
For 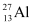 , the neutron removal reaction can be written as:
, the neutron removal reaction can be written as:
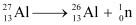
It is given that:
Mass 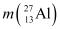 = 26.981541 u
= 26.981541 u
Mass 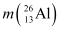 = 25.986895 u
= 25.986895 u
The mass defect of this reaction is given as:
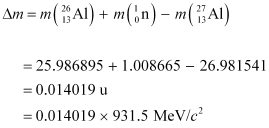
Hence, the energy required for neutron removal is calculated as:
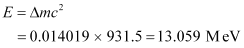
Q4: A source contains two phosphorous radio nuclides 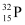 (T1/2 = 14.3d) and
(T1/2 = 14.3d) and 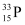 (T1/2 = 25.3d). Initially, 10% of the decays come from
(T1/2 = 25.3d). Initially, 10% of the decays come from  . How long one must wait until 90% do so?
. How long one must wait until 90% do so?
Ans: Half life of 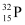 , T1/2 = 14.3 days
, T1/2 = 14.3 days
Half life of 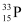 , T’1/2 = 25.3 days
, T’1/2 = 25.3 days
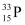 nucleus decay is 10% of the total amount of decay.
nucleus decay is 10% of the total amount of decay.
The source has initially 10% of 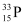 nucleus and 90% of
nucleus and 90% of 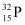 nucleus.
nucleus.
Suppose after t days, the source has 10% of 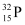 nucleus and 90% of
nucleus and 90% of  nucleus.
nucleus.
Initially:
Number of 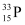 nucleus = N
nucleus = N
Number of 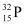 nucleus = 9 N
nucleus = 9 N
Finally:
Number of 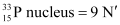
Number of 
For 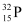 nucleus, we can write the number ratio as:
nucleus, we can write the number ratio as:
For 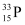 , we can write the number ratio as:
, we can write the number ratio as:

On dividing equation (1) by equation (2), we get:
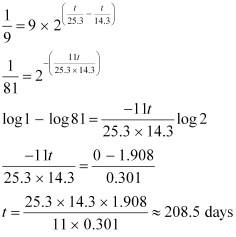
Hence, it will take about 208.5 days for 90% decay of 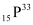 .
.
Q5: Under certain circumstances, a nucleus can decay by emitting a particle more massive than an α-particle. Consider the following decay processes:
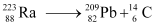
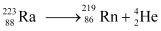
Calculate the Q-values for these decays and determine that both are energetically allowed.
Ans: Take a 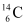 emission nuclear reaction:
emission nuclear reaction:
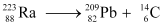
We know that:
Mass of 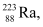 m1 = 223.01850 u
m1 = 223.01850 u
Mass of 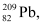 m2 = 208.98107 u
m2 = 208.98107 u
Mass of 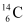 , m3 = 14.00324 u
, m3 = 14.00324 u
Hence, the Q-value of the reaction is given as:
Q = (m1 − m2 − m3) c2
= (223.01850 − 208.98107 − 14.00324) c2
= (0.03419 c2) u
But 1 u = 931.5 MeV/c2
∴Q = 0.03419 × 931.5
= 31.848 MeV
Hence, the Q-value of the nuclear reaction is 31.848 MeV. Since the value is positive, the reaction is energetically allowed.
Now take a 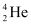 emission nuclear reaction:
emission nuclear reaction:
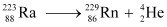
We know that:
Mass of 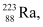 m1 = 223.01850
m1 = 223.01850
Mass of 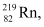 m2 = 219.00948
m2 = 219.00948
Mass of 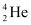 , m3 = 4.00260
, m3 = 4.00260
Q-value of this nuclear reaction is given as:
Q = (m1 − m2 − m3) c2
= (223.01850 − 219.00948 − 4.00260) C2
= (0.00642 c2) u
= 0.00642 × 931.5 = 5.98 MeV
Hence, the Q value of the second nuclear reaction is 5.98 MeV. Since the value is positive, the reaction is energetically allowed.
Q6: Consider the fission of 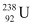 by fast neutrons. In one fission event, no neutrons are emitted and the final end products, after the beta decay of the primary fragments, are
by fast neutrons. In one fission event, no neutrons are emitted and the final end products, after the beta decay of the primary fragments, are 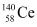 and
and 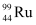 . Calculate Q for this fission process. The relevant atomic and particle masses are
. Calculate Q for this fission process. The relevant atomic and particle masses are
m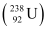 =238.05079 u
=238.05079 u
m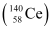 =139.90543 u
=139.90543 u
m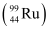 = 98.90594 u
= 98.90594 u
Ans: In the fission of 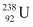 , 10 β− particles decay from the parent nucleus. The nuclear reaction can be written as:
, 10 β− particles decay from the parent nucleus. The nuclear reaction can be written as:
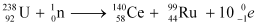
It is given that:
Mass of a nucleus 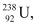 m1 = 238.05079 u
m1 = 238.05079 u
Mass of a nucleus 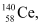 m2 = 139.90543 u
m2 = 139.90543 u
Mass of a nucleus 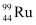 , m3 = 98.90594 u
, m3 = 98.90594 u
Mass of a neutron 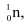 m4 = 1.008665 u
m4 = 1.008665 u
Q-value of the above equation,
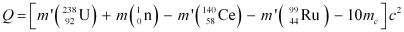
Where,
m’ = Represents the corresponding atomic masses of the nuclei
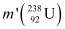 = m1 − 92me
= m1 − 92me
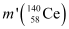 = m2 − 58me
= m2 − 58me
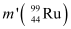 = m3 − 44me
= m3 − 44me
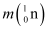 = m4
= m4
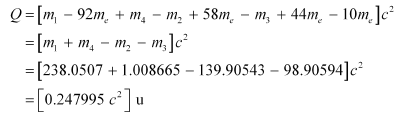
But 1 u = 931.5 MeV / c2
∴ O = 0.247995 x 931.5 = 23 1.007 MeV
Hence, the Q-value of the fission process is 231.007 MeV.
Q7: Consider the D−T reaction (deuterium−tritium fusion)
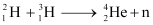
(a) Calculate the energy released in MeV in this reaction from the data:
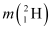 = 2.014102 u
= 2.014102 u
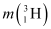 = 3.016049 u
= 3.016049 u
(b) Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction? (Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzmann’s constant, T = absolute temperature.)
Ans: (a) Take the D-T nuclear reaction: 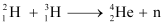
It is given that:
Mass of 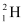 , m1= 2.014102 u
, m1= 2.014102 u
Mass of 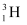 , m2 = 3.016049 u
, m2 = 3.016049 u
Mass of 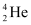 m3 = 4.002603 u
m3 = 4.002603 u
Mass of 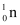 , m4 = 1.008665 u
, m4 = 1.008665 u
Q-value of the given D-T reaction is:
Q = [m1 + m2− m3 − m4] c2
= [2.014102 + 3.016049 − 4.002603 − 1.008665] c2
= [0.018883 c2] u
But 1 u = 931.5 MeV/c2
∴ Q = 0.018883 × 931.5 = 17.59 MeV
(b) Radius of deuterium and tritium, r ≈ 2.0 fm = 2 × 10−15 m
Distance between the two nuclei at the moment when they touch each other, d = r + r = 4 × 10−15 m
Charge on the deuterium nucleus = e
Charge on the tritium nucleus = e
Hence, the repulsive potential energy between the two nuclei is given as:
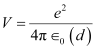
Where,
∈0 = Permittivity of free space
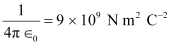
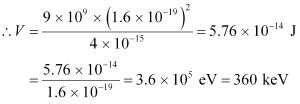
Hence, 5.76 × 10−14 J or 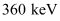 of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
However, it is given that:
KE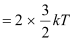
Where,
k = Boltzmann constant = 1.38 × 10−23 m2 kg s−2 K−1
T = Temperature required for triggering the reaction
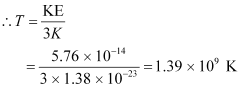
Hence, the gas must be heated to a temperature of 1.39 × 109 K to initiate the reaction.
Q8: Obtain the maximum kinetic energy of β-particles, and the radiation frequencies of γ decays in the decay scheme shown in Fig. 13.6. You are given that
m (198Au) = 197.968233 u
m (198Hg) =197.966760 u
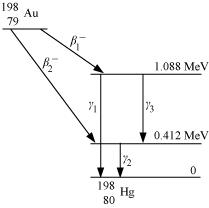
Ans: It can be observed from the given γ-decay diagram that γ1 decays from the 1.088 MeV energy level to the 0 MeV energy level.
Hence, the energy corresponding to γ1-decay is given as:
E1 = 1.088 − 0 = 1.088 MeV
hν1= 1.088 × 1.6 × 10−19 × 106 J
Where,
h = Planck’s constant = 6.6 × 10−34 Js
ν1 = Frequency of radiation radiated by γ1-decay
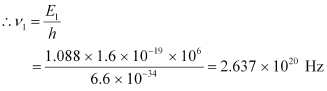
It can be observed from the given γ-decay diagram that γ2 decays from the 0.412 MeV energy level to the 0 MeV energy level.
Hence, the energy corresponding to γ2-decay is given as:
E2 = 0.412 − 0 = 0.412 MeV
hν2= 0.412 × 1.6 × 10−19 × 106 J
Where,
ν2 = Frequency of radiation radiated by γ2-decay
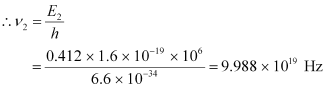
It can be observed from the given γ-decay diagram that γ3 decays from the 1.088 MeV energy level to the 0.412 MeV energy level.
Hence, the energy corresponding to γ3-decay is given as:
E3 = 1.088 − 0.412 = 0.676 MeV
hν3= 0.676 × 10−19 × 106 J
Where,
ν3 = Frequency of radiation radiated by γ3-decay
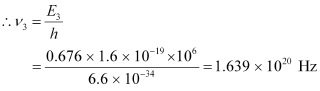
Mass of 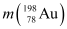 = 197.968233 u
= 197.968233 u
Mass of 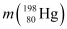 = 197.966760 u
= 197.966760 u
1 u = 931.5 MeV/c2
Energy of the highest level is given as:
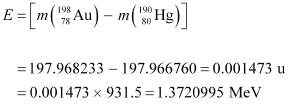
β1 decays from the 1.3720995 MeV level to the 1.088 MeV level
∴Maximum kinetic energy of the β1 particle = 1.3720995 − 1.088
= 0.2840995 MeV
β2 decays from the 1.3720995 MeV level to the 0.412 MeV level
∴Maximum kinetic energy of the β2 particle = 1.3720995 − 0.412
= 0.9600995 MeV
Q9: Calculate and compare the energy released by a) fusion of 1.0 kg of hydrogen deep within Sun and b) the fission of 1.0 kg of 235U in a fission reactor.
Ans: (a) Amount of hydrogen, m = 1 kg = 1000 g
1 mole, i.e., 1 g of hydrogen (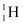 ) contains 6.023 × 1023 atoms.
) contains 6.023 × 1023 atoms.
∴1000 g of 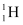 contains 6.023 × 1023 × 1000 atoms.
contains 6.023 × 1023 × 1000 atoms.
Within the sun, four 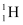 nuclei combine and form one
nuclei combine and form one 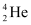 nucleus. In this process 26 MeV of energy is released.
nucleus. In this process 26 MeV of energy is released.
Hence, the energy released from the fusion of 1 kg 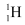 is:
is:
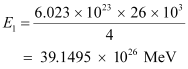
(b) Amount of  = 1 kg = 1000 g
= 1 kg = 1000 g
1 mole, i.e., 235 g of  contains 6.023 × 1023 atoms.
contains 6.023 × 1023 atoms.
∴1000 g of  contains
contains 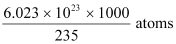
It is known that the amount of energy released in the fission of one atom of 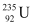 is 200 MeV.
is 200 MeV.
Hence, energy released from the fission of 1 kg of 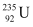 is:
is:

∴ 
Therefore, the energy released in the fusion of 1 kg of hydrogen is nearly 8 times the energy released in the fission of 1 kg of uranium.
Q10: Suppose India had a target of producing by 2020 AD, 200,000 MW of electric power, ten percent of which was to be obtained from nuclear power plants. Suppose we are given that, on an average, the efficiency of utilization (i.e. conversion to electric energy) of thermal energy produced in a reactor was 25%. How much amount of fissionable uranium would our country need per year by 2020? Take the heat energy per fission of 235U to be about 200MeV.
Ans: Amount of electric power to be generated, P = 2 × 105 MW
10% of this amount has to be obtained from nuclear power plants.
∴ Amount of nuclear power, 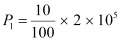
= 2 × 104 MW
= 2 × 104 × 106 J/s
= 2 × 1010 × 60 × 60 × 24 × 365 J/y
Heat energy released per fission of a 235U nucleus, E = 200 MeV
Efficiency of a reactor = 25%
Hence, the amount of energy converted into the electrical energy per fission is calculated as:
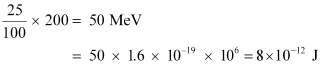
Number of atoms required for fission per year:
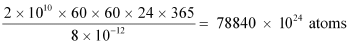
1 mole, i.e., 235 g of U235 contains 6.023 × 1023 atoms.
∴ Mass of 6.023 × 1023 atoms of U235 = 235 g = 235 × 10−3 kg
∴ Mass of 78840 × 1024 atoms of U235
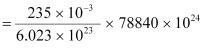
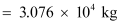
Hence, the mass of uranium needed per year is 3.076 × 104 kg.
Q11: (a) Two stable isotopes of lithium 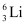 and
and 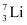 have respective abundances of 7.5% and 92.5%. These isotopes have masses 6.01512 u and 7.01600 u, respectively. Find the atomic mass of lithium.
have respective abundances of 7.5% and 92.5%. These isotopes have masses 6.01512 u and 7.01600 u, respectively. Find the atomic mass of lithium.
(b) Boron has two stable isotopes, 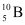 and
and 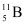 . Their respective masses are 10.01294 u and 11.00931 u, and the atomic mass of boron is 10.811 u. Find the abundances of
. Their respective masses are 10.01294 u and 11.00931 u, and the atomic mass of boron is 10.811 u. Find the abundances of 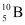 and
and 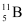 .
.
Ans: (a) Mass of lithium isotope 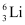 , m1 = 6.01512 u
, m1 = 6.01512 u
Mass of lithium isotope 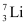 , m2 = 7.01600 u
, m2 = 7.01600 u
Abundance of 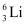 , η1= 7.5%
, η1= 7.5%
Abundance of 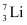 , η2= 92.5%
, η2= 92.5%
The atomic mass of lithium atom is given as:
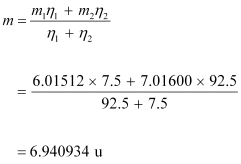
(b) Mass of boron isotope 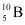 , m1 = 10.01294 u
, m1 = 10.01294 u
Mass of boron isotope 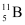 , m2 = 11.00931 u
, m2 = 11.00931 u
Abundance of 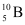 , η1 = x%
, η1 = x%
Abundance of 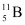 , η2= (100 − x)%
, η2= (100 − x)%
Atomic mass of boron, m = 10.811 u
The atomic mass of boron atom is given as:
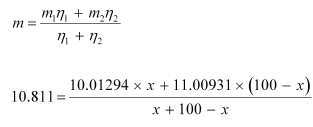
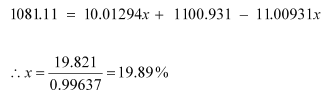
And 100 − x = 80.11%
Hence, the abundance of 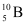 is 19.89% and that of
is 19.89% and that of 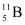 is 80.11%.
is 80.11%.
Q12: The three stable isotopes of neon: 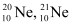 and
and 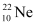 have respective abundances of 90.51%, 0.27% and 9.22%. The atomic masses of the three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.
have respective abundances of 90.51%, 0.27% and 9.22%. The atomic masses of the three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.
Ans: Atomic mass of 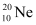 , m1= 19.99 u
, m1= 19.99 u
Abundance of 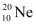 , η1 = 90.51%
, η1 = 90.51%
Atomic mass of 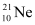 , m2 = 20.99 u
, m2 = 20.99 u
Abundance of 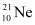 , η2 = 0.27%
, η2 = 0.27%
Atomic mass of 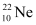 , m3 = 21.99 u
, m3 = 21.99 u
Abundance of 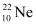 , η3 = 9.22%
, η3 = 9.22%
The average atomic mass of neon is given as:
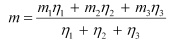
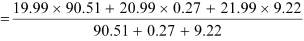
= 20.1771 u
Q13: Write nuclear reaction equations for
(i) α-decay of 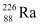
(ii) α-decay of 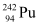
(iii) β−-decay of 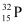
(iv) β−-decay of 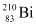
(v) β -decay of 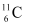
(vi) β -decay of 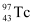
(vii) Electron capture of 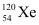
Ans: α is a nucleus of helium 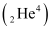 and β is an electron (e− for β− and e for β ). In every α-decay, there is a loss of 2 protons and 4 neutrons. In every β -decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β−-decay, there is a gain of 1 proton and an anti-neutrino is emitted from the nucleus.
and β is an electron (e− for β− and e for β ). In every α-decay, there is a loss of 2 protons and 4 neutrons. In every β -decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β−-decay, there is a gain of 1 proton and an anti-neutrino is emitted from the nucleus.
For the given cases, the various nuclear reactions can be written as:

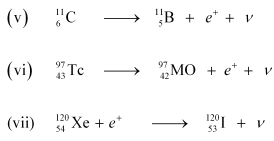
Q14: A radioactive isotope has a half-life of T years. How long will it take the activity to reduce to a) 3.125%, b) 1% of its original value?
Ans: Half-life of the radioactive isotope = T years
Original amount of the radioactive isotope = N0
(a) After decay, the amount of the radioactive isotope = N
It is given that only 3.125% of N0 remains after decay. Hence, we can write:
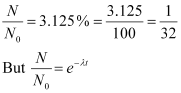
Where,
λ = Decay constant
t = Time
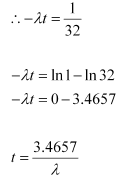
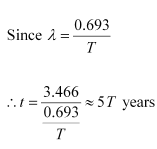
Hence, the isotope will take about 5T years to reduce to 3.125% of its original value.
(b) After decay, the amount of the radioactive isotope = N
It is given that only 1% of N0 remains after decay. Hence, we can write:
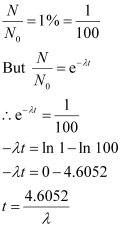
Since, λ = 0.693/T
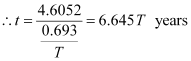
Hence, the isotope will take about 6.645 T years to reduce to 1% of its original value.
Q15: The normal activity of living carbon-containing matter is found to be about 15 decays per minute for every gram of carbon. This activity arises from the small proportion of radioactive 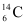 present with the stable carbon isotope
present with the stable carbon isotope 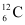 . When the organism is dead, its interaction with the atmosphere (which maintains the above equilibrium activity) ceases and its activity begins to drop. From the known half-life (5730 years) of
. When the organism is dead, its interaction with the atmosphere (which maintains the above equilibrium activity) ceases and its activity begins to drop. From the known half-life (5730 years) of 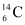 , and the measured activity, the age of the specimen can be approximately estimated. This is the principle of
, and the measured activity, the age of the specimen can be approximately estimated. This is the principle of 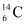 dating used in archaeology. Suppose a specimen from Mohenjodaro gives an activity of 9 decays per minute per gram of carbon. Estimate the approximate age of the Indus-Valley civilization.
dating used in archaeology. Suppose a specimen from Mohenjodaro gives an activity of 9 decays per minute per gram of carbon. Estimate the approximate age of the Indus-Valley civilization.
Ans: Decay rate of living carbon-containing matter, R = 15 decay/min
Let N be the number of radioactive atoms present in a normal carbon- containing matter.
Half life of 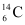 ,
, 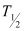 = 5730 years
= 5730 years
The decay rate of the specimen obtained from the Mohenjodaro site:
R' = 9 decays/min
Let N' be the number of radioactive atoms present in the specimen during the
ohenjodaro period.
Therefore, we can relate the decay constant, λand time, t as:
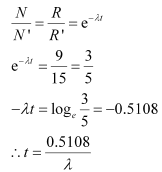
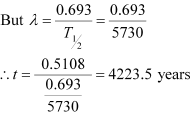
Hence, the approximate age of the Indus-Valley civilisation is 4223.5 years.
Q16: Obtain the amount of 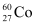 necessary to provide a radioactive source of 8.0 mCi strength. The half-life of
necessary to provide a radioactive source of 8.0 mCi strength. The half-life of 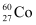 is 5.3 years.
is 5.3 years.
Ans: The strength of the radioactive source is given as:
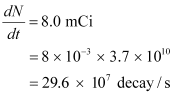
Where,
N = Required number of atoms
Half-life of 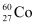 ,
, 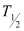 = 5.3 years
= 5.3 years
= 5.3 × 365 × 24 × 60 × 60
= 1.67 × 108 s
For decay constant λ, we have the rate of decay as:
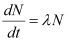
Where, λ 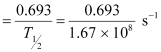
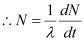
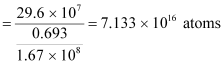
For 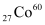 :
:
Mass of 6.023 × 1023 (Avogadro’s number) atoms = 60 g
∴Mass of 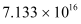 atoms
atoms 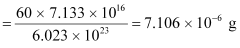
Hence, the amount of 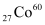 necessary for the purpose is 7.106 × 10−6 g.
necessary for the purpose is 7.106 × 10−6 g.
Q17: The half-life of 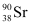 is 28 years. What is the disintegration rate of 15 mg of this isotope?
is 28 years. What is the disintegration rate of 15 mg of this isotope?
Ans: Half life of 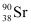 ,
, 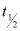 = 28 years
= 28 years
= 28 × 365 × 24 × 60 × 60
= 8.83 × 108 s
Mass of the isotope, m = 15 mg
90 g of 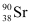 atom contains 6.023 × 1023 (Avogadro’s number) atoms.
atom contains 6.023 × 1023 (Avogadro’s number) atoms.
Therefore, 15 mg of 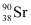 contains:
contains:
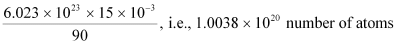
Rate of disintegration, 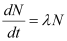
Where,
λ = Decay constant 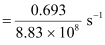
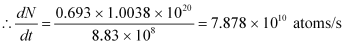
Hence, the disintegration rate of 15 mg of the given isotope is 7.878 × 1010 atoms/s.
Q18: Find the Q-value and the kinetic energy of the emitted α-particle in the α-decay of (a) 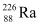 and (b)
and (b) 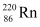 .
.
Given 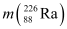 = 226.02540 u,
= 226.02540 u,
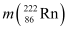 = 222.01750 u,
= 222.01750 u,
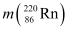 = 220.01137 u,
= 220.01137 u,
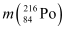 = 216.00189 u.
= 216.00189 u.
Ans: (a) Alpha particle decay of 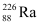 emits a helium nucleus. As a result, its mass number reduces to (226 − 4) 222 and its atomic number reduces to (88 − 2) 86. This is shown in the following nuclear reaction.
emits a helium nucleus. As a result, its mass number reduces to (226 − 4) 222 and its atomic number reduces to (88 − 2) 86. This is shown in the following nuclear reaction.
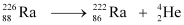
Q-value of emitted α-particle = (Sum of initial mass − Sum of final mass) c2
Where,
c = Speed of light
It is given that:
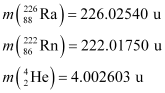
Q-value = [226.02540 − (222.01750 4.002603)] u c2
= 0.005297 u c2
But 1 u = 931.5 MeV/c2
∴Q = 0.005297 × 931.5 ≈ 4.94 MeV
Kinetic energy of the α-particle 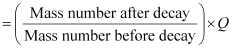
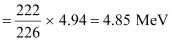
(b) Alpha particle decay of 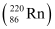 is shown by the following nuclear reaction.
is shown by the following nuclear reaction.
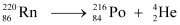
It is given that:
Mass of 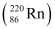 = 220.01137 u
= 220.01137 u
Mass of 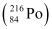 = 216.00189 u
= 216.00189 u
∴Q-value = 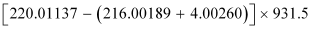
≈ 641 MeV
Kinetic energy of the α-particle 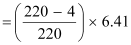
= 6.29 MeV
Q19: The radionuclide 11C decays according to

The maximum energy of the emitted positron is 0.960 MeV.
Given the mass values:
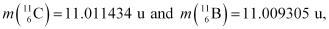
calculate Q and compare it with the maximum energy of the positron emitted
Ans: The given nuclear reaction is:
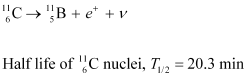
Atomic mass of 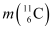 = 11.011434 u
= 11.011434 u
Atomic mass of 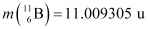
Maximum energy possessed by the emitted positron = 0.960 MeV
The change in the Q-value (ΔQ) of the nuclear masses of the 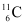 nucleus is given as:
nucleus is given as:
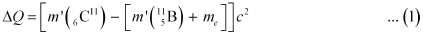
Where,
me = Mass of an electron or positron = 0.000548 u
c = Speed of light
m’ = Respective nuclear masses
If atomic masses are used instead of nuclear masses, then we have to add 6 me in the case of 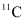 and 5 me in the case of
and 5 me in the case of 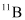 .
.
Hence, equation (1) reduces to:
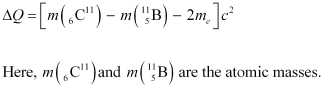
∴ΔQ = [11.011434 − 11.009305 − 2 × 0.000548] c2
= (0.001033 c2) u
But 1 u = 931.5 Mev/c2
∴ΔQ = 0.001033 × 931.5 ≈ 0.962 MeV
The value of Q is almost comparable to the maximum energy of the emitted positron.
Q20: The nucleus 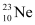 decays by
decays by 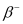 emission. Write down the
emission. Write down the 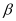 decay equation and determine the maximum kinetic energy of the electrons emitted. Given that:
decay equation and determine the maximum kinetic energy of the electrons emitted. Given that:
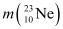 = 22.994466 u
= 22.994466 u
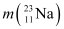 = 22.989770 u.
= 22.989770 u.
Ans: In 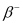 emission, the number of protons increases by 1, and one electron and an antineutrino are emitted from the parent nucleus.
emission, the number of protons increases by 1, and one electron and an antineutrino are emitted from the parent nucleus.
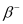 emission of the nucleus
emission of the nucleus 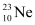 is given as:
is given as:
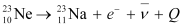
It is given that:
Atomic mass of 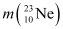 = 22.994466 u
= 22.994466 u
Atomic mass of 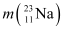 = 22.989770 u
= 22.989770 u
Mass of an electron, me = 0.000548 u
Q-value of the given reaction is given as:
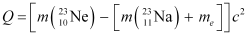
There are 10 electrons in 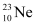 and 11 electrons in
and 11 electrons in 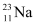 . Hence, the mass of the electron is cancelled in the Q-value equation.
. Hence, the mass of the electron is cancelled in the Q-value equation.
∴ Q = [ 22.994466 - 22.989770]c2
= (0.004696 c2) u
But 1 u = 931.5 Me V/c2
∴ Q = 0.004696 x 931.5 = 4.374 Me V
The daughter nucleus is too heavy as compared to 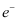 and
and 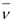 . Hence, it carries negligible energy. The kinetic energy of the antineutrino is nearly zero. Hence, the maximum kinetic energy of the emitted electrons is almost equal to the Q-value, i.e., 4.374 MeV.
. Hence, it carries negligible energy. The kinetic energy of the antineutrino is nearly zero. Hence, the maximum kinetic energy of the emitted electrons is almost equal to the Q-value, i.e., 4.374 MeV.
Q21: A 1000 MW fission reactor consumes half of its fuel in 5.00 y. How much 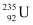 did it contain initially? Assume that the reactor operates 80% of the time, that all the energy generated arises from the fission of
did it contain initially? Assume that the reactor operates 80% of the time, that all the energy generated arises from the fission of 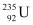 and that this nuclide is consumed only by the fission process.
and that this nuclide is consumed only by the fission process.
Ans: Half life of the fuel of the fission reactor,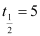 years
years
= 5 × 365 × 24 × 60 × 60 s
We know that in the fission of 1 g of 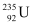 nucleus, the energy released is equal to 200 MeV.
nucleus, the energy released is equal to 200 MeV.
1 mole, i.e., 235 g of 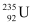 contains 6.023 × 1023 atoms.
contains 6.023 × 1023 atoms.
∴1 g 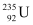 contains
contains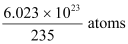
The total energy generated per gram of 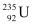 is calculated as:
is calculated as:
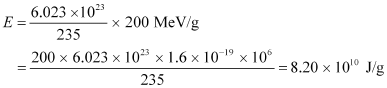
The reactor operates only 80% of the time.
Hence, the amount of 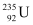 consumed in 5 years by the 1000 MW fission reactor is calculated as:
consumed in 5 years by the 1000 MW fission reactor is calculated as:
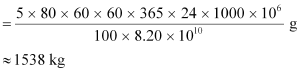
∴ Initial amount of 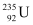 = 2 × 1538 = 3076 kg.
= 2 × 1538 = 3076 kg.
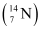 , given
, given 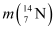 =14.00307 u
=14.00307 u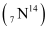 , m = 14.00307 u
, m = 14.00307 u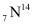 contains 7 protons and 7 neutrons.
contains 7 protons and 7 neutrons.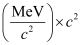
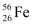 and
and 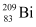 in units of MeV from the following data:
in units of MeV from the following data: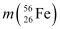 = 55.934939 u ,
= 55.934939 u , 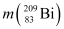 = 208.980388 u
= 208.980388 u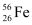 , m1 = 55.934939 u
, m1 = 55.934939 u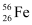 nucleus has 26 protons and (56 − 26) = 30 neutrons
nucleus has 26 protons and (56 − 26) = 30 neutrons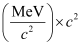
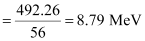
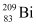 , m2 = 208.980388 u
, m2 = 208.980388 u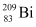 nucleus has 83 protons and (209 − 83) 126 neutrons.
nucleus has 83 protons and (209 − 83) 126 neutrons.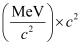
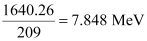
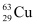 atoms (of mass 62.92960 u).
atoms (of mass 62.92960 u).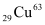 atom, m = 62.92960 u
atom, m = 62.92960 u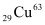 atoms in the coin
atoms in the coin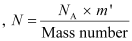
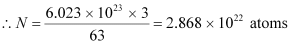
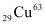 nucleus has 29 protons and (63 − 29) 34 neutrons
nucleus has 29 protons and (63 − 29) 34 neutrons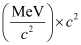
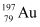 and the silver isotope
and the silver isotope 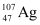 .
.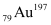 = RAu
= RAu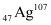 = RAg
= RAg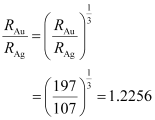
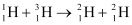
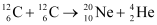
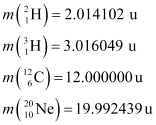
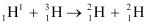
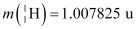
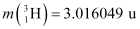
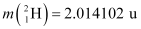
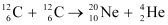
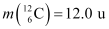
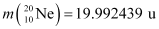
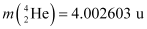
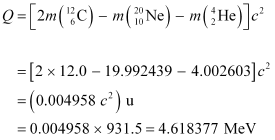
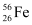 nucleus into two equal fragments,
nucleus into two equal fragments, 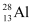 . Is the fission energetically possible? Argue by working out Q of the process. Given
. Is the fission energetically possible? Argue by working out Q of the process. Given 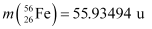 and
and 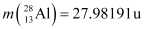 .
.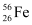 can be given as:
can be given as: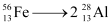
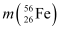 = 55.93494 u
= 55.93494 u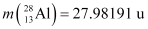
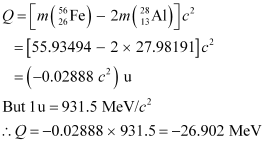
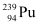 are very similar to those of
are very similar to those of 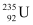 . The average energy released per fission is 180 MeV. How much energy, in MeV, is released if all the atoms in 1 kg of pure
. The average energy released per fission is 180 MeV. How much energy, in MeV, is released if all the atoms in 1 kg of pure 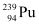 undergo fission?
undergo fission?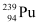 ,
, 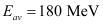
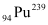 , m = 1 kg = 1000 g
, m = 1 kg = 1000 g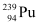 = 239 g
= 239 g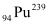 contains NA atoms.
contains NA atoms.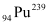 contains
contains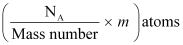
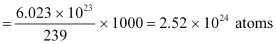
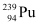 is calculated as:-
is calculated as:-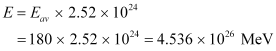
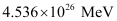 is released if all the atoms in 1 kg of pure
is released if all the atoms in 1 kg of pure 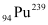 undergo fission.
undergo fission.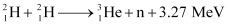
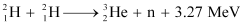
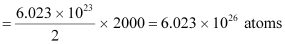
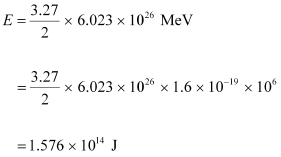
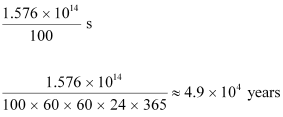
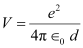
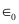 = Permittivity of free space
= Permittivity of free space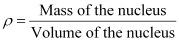
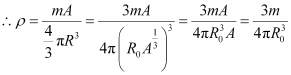
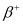 (positron) emission from a nucleus, there is another competing process known as electron capture (electron from an inner orbit, say, the K−shell, is captured by the nucleus and a neutrino is emitted).
(positron) emission from a nucleus, there is another competing process known as electron capture (electron from an inner orbit, say, the K−shell, is captured by the nucleus and a neutrino is emitted).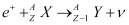
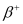 emission is energetically allowed, electron capture is necessarily allowed but not vice−versa.
emission is energetically allowed, electron capture is necessarily allowed but not vice−versa.

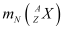 = Nuclear mass of
= Nuclear mass of 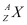
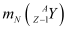 = Nuclear mass of
= Nuclear mass of 
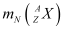 = Atomic mass of
= Atomic mass of 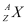
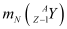 = Atomic mass of
= Atomic mass of 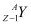
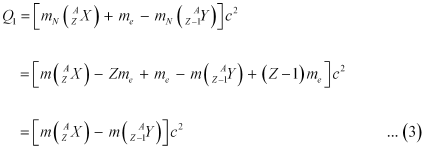
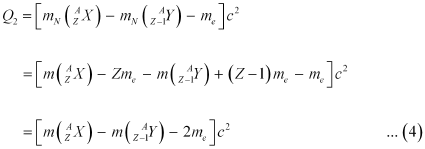
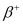 emission is energetically allowed, then the electron capture process is necessarily allowed, but not vice-versa. This is because the Q-value must be positive for an energetically-allowed nuclear reaction.
emission is energetically allowed, then the electron capture process is necessarily allowed, but not vice-versa. This is because the Q-value must be positive for an energetically-allowed nuclear reaction.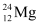 (23.98504u),
(23.98504u), 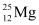 (24.98584u) and
(24.98584u) and 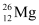 (25.98259u). The natural abundance of
(25.98259u). The natural abundance of 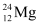 is 78.99% by mass. Calculate the abundances of other two isotopes.
is 78.99% by mass. Calculate the abundances of other two isotopes.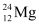 , m1 = 23.98504 u
, m1 = 23.98504 u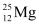 , m2 = 24.98584 u
, m2 = 24.98584 u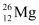 , m3 = 25.98259 u
, m3 = 25.98259 u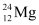 , η1= 78.99%
, η1= 78.99%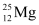 , η2 = x%
, η2 = x%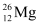 , η3 = 100 − x − 78.99% = (21.01 − x)%
, η3 = 100 − x − 78.99% = (21.01 − x)%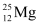 is 9.3% and that of
is 9.3% and that of 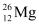 is 11.71%.
is 11.71%.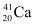 and
and 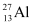 from the following data:
from the following data: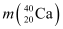 = 39.962591 u
= 39.962591 u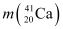 = 40.962278 u
= 40.962278 u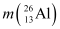 = 25.986895 u
= 25.986895 u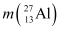 = 26.981541 u
= 26.981541 u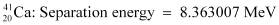
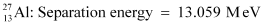
 is removed from a
is removed from a 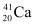 nucleus. The corresponding nuclear reaction can be written as:
nucleus. The corresponding nuclear reaction can be written as: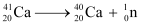
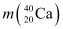 = 39.962591 u
= 39.962591 u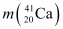 = 40.962278 u
= 40.962278 u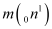 = 1.008665 u
= 1.008665 u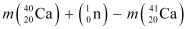
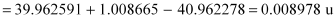
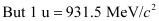
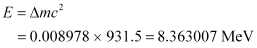
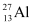 , the neutron removal reaction can be written as:
, the neutron removal reaction can be written as: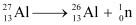
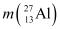 = 26.981541 u
= 26.981541 u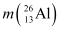 = 25.986895 u
= 25.986895 u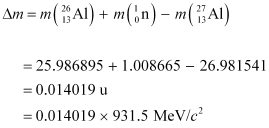
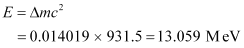
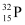 (T1/2 = 14.3d) and
(T1/2 = 14.3d) and 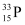 (T1/2 = 25.3d). Initially, 10% of the decays come from
(T1/2 = 25.3d). Initially, 10% of the decays come from  . How long one must wait until 90% do so?
. How long one must wait until 90% do so?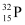 , T1/2 = 14.3 days
, T1/2 = 14.3 days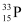 , T’1/2 = 25.3 days
, T’1/2 = 25.3 days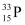 nucleus decay is 10% of the total amount of decay.
nucleus decay is 10% of the total amount of decay.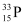 nucleus and 90% of
nucleus and 90% of 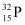 nucleus.
nucleus.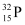 nucleus and 90% of
nucleus and 90% of  nucleus.
nucleus.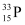 nucleus = N
nucleus = N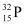 nucleus = 9 N
nucleus = 9 N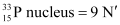

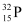 nucleus, we can write the number ratio as:
nucleus, we can write the number ratio as:
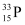 , we can write the number ratio as:
, we can write the number ratio as:
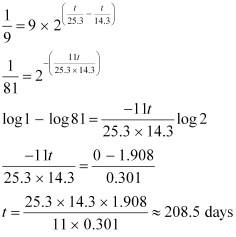
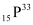 .
.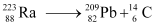
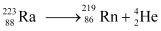
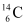 emission nuclear reaction:
emission nuclear reaction: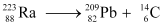
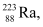 m1 = 223.01850 u
m1 = 223.01850 u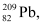 m2 = 208.98107 u
m2 = 208.98107 u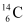 , m3 = 14.00324 u
, m3 = 14.00324 u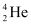 emission nuclear reaction:
emission nuclear reaction: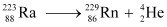
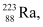 m1 = 223.01850
m1 = 223.01850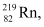 m2 = 219.00948
m2 = 219.00948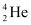 , m3 = 4.00260
, m3 = 4.00260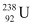 by fast neutrons. In one fission event, no neutrons are emitted and the final end products, after the beta decay of the primary fragments, are
by fast neutrons. In one fission event, no neutrons are emitted and the final end products, after the beta decay of the primary fragments, are 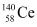 and
and 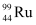 . Calculate Q for this fission process. The relevant atomic and particle masses are
. Calculate Q for this fission process. The relevant atomic and particle masses are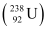 =238.05079 u
=238.05079 u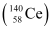 =139.90543 u
=139.90543 u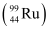 = 98.90594 u
= 98.90594 u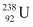 , 10 β− particles decay from the parent nucleus. The nuclear reaction can be written as:
, 10 β− particles decay from the parent nucleus. The nuclear reaction can be written as: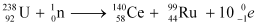
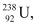 m1 = 238.05079 u
m1 = 238.05079 u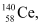 m2 = 139.90543 u
m2 = 139.90543 u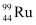 , m3 = 98.90594 u
, m3 = 98.90594 u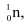 m4 = 1.008665 u
m4 = 1.008665 u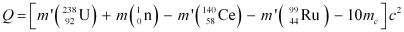
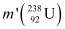 = m1 − 92me
= m1 − 92me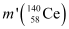 = m2 − 58me
= m2 − 58me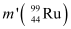 = m3 − 44me
= m3 − 44me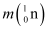 = m4
= m4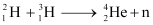
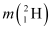 = 2.014102 u
= 2.014102 u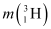 = 3.016049 u
= 3.016049 u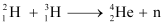
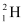 , m1= 2.014102 u
, m1= 2.014102 u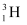 , m2 = 3.016049 u
, m2 = 3.016049 u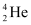 m3 = 4.002603 u
m3 = 4.002603 u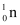 , m4 = 1.008665 u
, m4 = 1.008665 u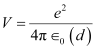
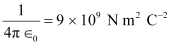
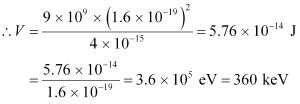
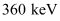 of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.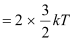
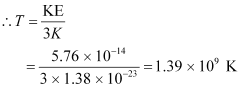

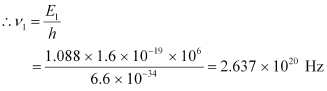
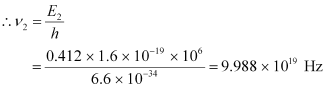
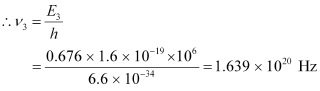
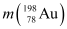 = 197.968233 u
= 197.968233 u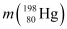 = 197.966760 u
= 197.966760 u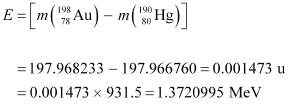
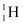 ) contains 6.023 × 1023 atoms.
) contains 6.023 × 1023 atoms.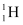 contains 6.023 × 1023 × 1000 atoms.
contains 6.023 × 1023 × 1000 atoms.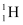 nuclei combine and form one
nuclei combine and form one 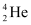 nucleus. In this process 26 MeV of energy is released.
nucleus. In this process 26 MeV of energy is released.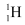 is:
is: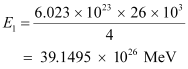
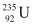 = 1 kg = 1000 g
= 1 kg = 1000 g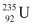 contains 6.023 × 1023 atoms.
contains 6.023 × 1023 atoms.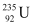 contains
contains 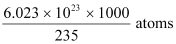
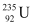 is 200 MeV.
is 200 MeV.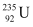 is:
is:

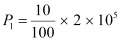
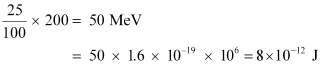
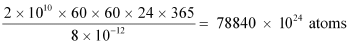
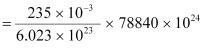
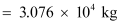
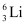 and
and 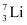 have respective abundances of 7.5% and 92.5%. These isotopes have masses 6.01512 u and 7.01600 u, respectively. Find the atomic mass of lithium.
have respective abundances of 7.5% and 92.5%. These isotopes have masses 6.01512 u and 7.01600 u, respectively. Find the atomic mass of lithium.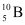 and
and 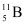 . Their respective masses are 10.01294 u and 11.00931 u, and the atomic mass of boron is 10.811 u. Find the abundances of
. Their respective masses are 10.01294 u and 11.00931 u, and the atomic mass of boron is 10.811 u. Find the abundances of 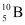 and
and 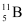 .
.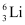 , m1 = 6.01512 u
, m1 = 6.01512 u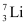 , m2 = 7.01600 u
, m2 = 7.01600 u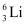 , η1= 7.5%
, η1= 7.5%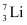 , η2= 92.5%
, η2= 92.5%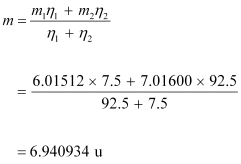
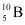 , m1 = 10.01294 u
, m1 = 10.01294 u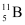 , m2 = 11.00931 u
, m2 = 11.00931 u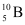 , η1 = x%
, η1 = x%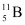 , η2= (100 − x)%
, η2= (100 − x)%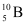 is 19.89% and that of
is 19.89% and that of 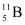 is 80.11%.
is 80.11%.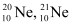 and
and 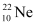 have respective abundances of 90.51%, 0.27% and 9.22%. The atomic masses of the three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.
have respective abundances of 90.51%, 0.27% and 9.22%. The atomic masses of the three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.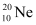 , m1= 19.99 u
, m1= 19.99 u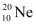 , η1 = 90.51%
, η1 = 90.51%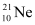 , m2 = 20.99 u
, m2 = 20.99 u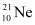 , η2 = 0.27%
, η2 = 0.27%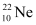 , m3 = 21.99 u
, m3 = 21.99 u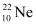 , η3 = 9.22%
, η3 = 9.22%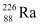
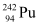
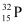
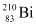
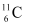
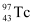
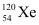
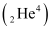 and β is an electron (e− for β− and e for β ). In every α-decay, there is a loss of 2 protons and 4 neutrons. In every β -decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β−-decay, there is a gain of 1 proton and an anti-neutrino is emitted from the nucleus.
and β is an electron (e− for β− and e for β ). In every α-decay, there is a loss of 2 protons and 4 neutrons. In every β -decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β−-decay, there is a gain of 1 proton and an anti-neutrino is emitted from the nucleus.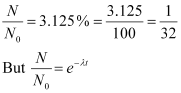
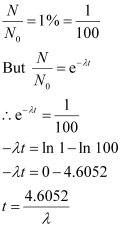
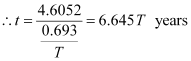
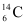 present with the stable carbon isotope
present with the stable carbon isotope 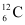 . When the organism is dead, its interaction with the atmosphere (which maintains the above equilibrium activity) ceases and its activity begins to drop. From the known half-life (5730 years) of
. When the organism is dead, its interaction with the atmosphere (which maintains the above equilibrium activity) ceases and its activity begins to drop. From the known half-life (5730 years) of 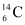 , and the measured activity, the age of the specimen can be approximately estimated. This is the principle of
, and the measured activity, the age of the specimen can be approximately estimated. This is the principle of 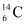 dating used in archaeology. Suppose a specimen from Mohenjodaro gives an activity of 9 decays per minute per gram of carbon. Estimate the approximate age of the Indus-Valley civilization.
dating used in archaeology. Suppose a specimen from Mohenjodaro gives an activity of 9 decays per minute per gram of carbon. Estimate the approximate age of the Indus-Valley civilization. ,
, 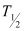 = 5730 years
= 5730 years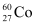 necessary to provide a radioactive source of 8.0 mCi strength. The half-life of
necessary to provide a radioactive source of 8.0 mCi strength. The half-life of 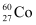 is 5.3 years.
is 5.3 years.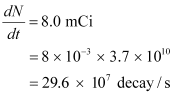
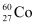 ,
, 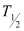 = 5.3 years
= 5.3 years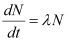
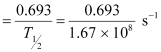
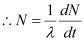
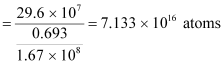
 :
: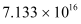 atoms
atoms 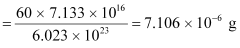
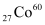 necessary for the purpose is 7.106 × 10−6 g.
necessary for the purpose is 7.106 × 10−6 g.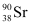 is 28 years. What is the disintegration rate of 15 mg of this isotope?
is 28 years. What is the disintegration rate of 15 mg of this isotope?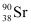 ,
, 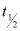 = 28 years
= 28 years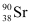 atom contains 6.023 × 1023 (Avogadro’s number) atoms.
atom contains 6.023 × 1023 (Avogadro’s number) atoms.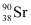 contains:
contains: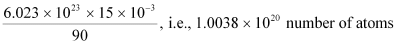
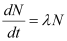
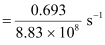
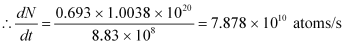
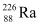 and (b)
and (b) 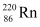 .
.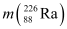 = 226.02540 u,
= 226.02540 u, 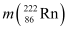 = 222.01750 u,
= 222.01750 u,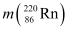 = 220.01137 u,
= 220.01137 u,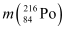 = 216.00189 u.
= 216.00189 u.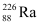 emits a helium nucleus. As a result, its mass number reduces to (226 − 4) 222 and its atomic number reduces to (88 − 2) 86. This is shown in the following nuclear reaction.
emits a helium nucleus. As a result, its mass number reduces to (226 − 4) 222 and its atomic number reduces to (88 − 2) 86. This is shown in the following nuclear reaction.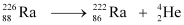
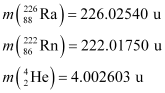
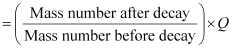
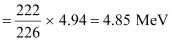
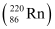 is shown by the following nuclear reaction.
is shown by the following nuclear reaction.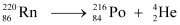
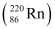 = 220.01137 u
= 220.01137 u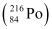 = 216.00189 u
= 216.00189 u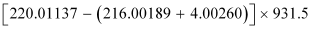
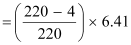

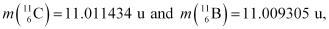
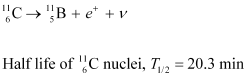
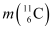 = 11.011434 u
= 11.011434 u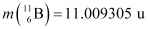
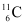 nucleus is given as:
nucleus is given as: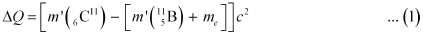
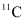 and 5 me in the case of
and 5 me in the case of 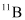 .
.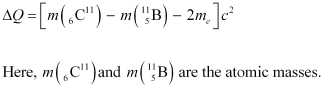
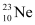 decays by
decays by 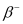 emission. Write down the
emission. Write down the 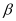 decay equation and determine the maximum kinetic energy of the electrons emitted. Given that:
decay equation and determine the maximum kinetic energy of the electrons emitted. Given that: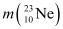 = 22.994466 u
= 22.994466 u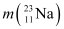 = 22.989770 u.
= 22.989770 u.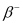 emission, the number of protons increases by 1, and one electron and an antineutrino are emitted from the parent nucleus.
emission, the number of protons increases by 1, and one electron and an antineutrino are emitted from the parent nucleus.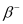 emission of the nucleus
emission of the nucleus 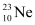 is given as:
is given as: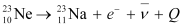
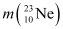 = 22.994466 u
= 22.994466 u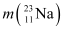 = 22.989770 u
= 22.989770 u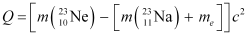
 and 11 electrons in
and 11 electrons in  . Hence, the mass of the electron is cancelled in the Q-value equation.
. Hence, the mass of the electron is cancelled in the Q-value equation. and
and 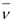 . Hence, it carries negligible energy. The kinetic energy of the antineutrino is nearly zero. Hence, the maximum kinetic energy of the emitted electrons is almost equal to the Q-value, i.e., 4.374 MeV.
. Hence, it carries negligible energy. The kinetic energy of the antineutrino is nearly zero. Hence, the maximum kinetic energy of the emitted electrons is almost equal to the Q-value, i.e., 4.374 MeV.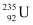 did it contain initially? Assume that the reactor operates 80% of the time, that all the energy generated arises from the fission of
did it contain initially? Assume that the reactor operates 80% of the time, that all the energy generated arises from the fission of 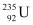 and that this nuclide is consumed only by the fission process.
and that this nuclide is consumed only by the fission process.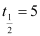 years
years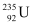 nucleus, the energy released is equal to 200 MeV.
nucleus, the energy released is equal to 200 MeV.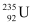 contains 6.023 × 1023 atoms.
contains 6.023 × 1023 atoms.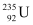 contains
contains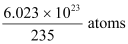
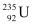 is calculated as:
is calculated as: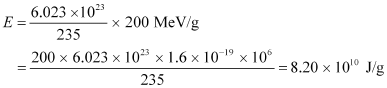
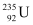 consumed in 5 years by the 1000 MW fission reactor is calculated as:
consumed in 5 years by the 1000 MW fission reactor is calculated as: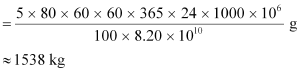
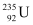 = 2 × 1538 = 3076 kg.
= 2 × 1538 = 3076 kg.


























