NCERT Solutions for Class 10 Science Chapter 3 - Metals and Non-metals
| Table of contents |

|
| Page No. 40 |

|
| Page No. 46 |

|
| Page No. 49 |

|
| Page No. 53 |

|
| Page No. 55 |

|
| Excercise (Page 56) |

|
| Page No. 57 |

|
Page No. 40
Q1. Give an example of a metal which
(a) is a liquid at room temperature.
(b) can be easily cut with a knife.
(c) is the best conductor of heat.
(d) is a poor conductor of heat.
Ans:
(a) Metal that exists in a liquid state at room temperature is mercury.
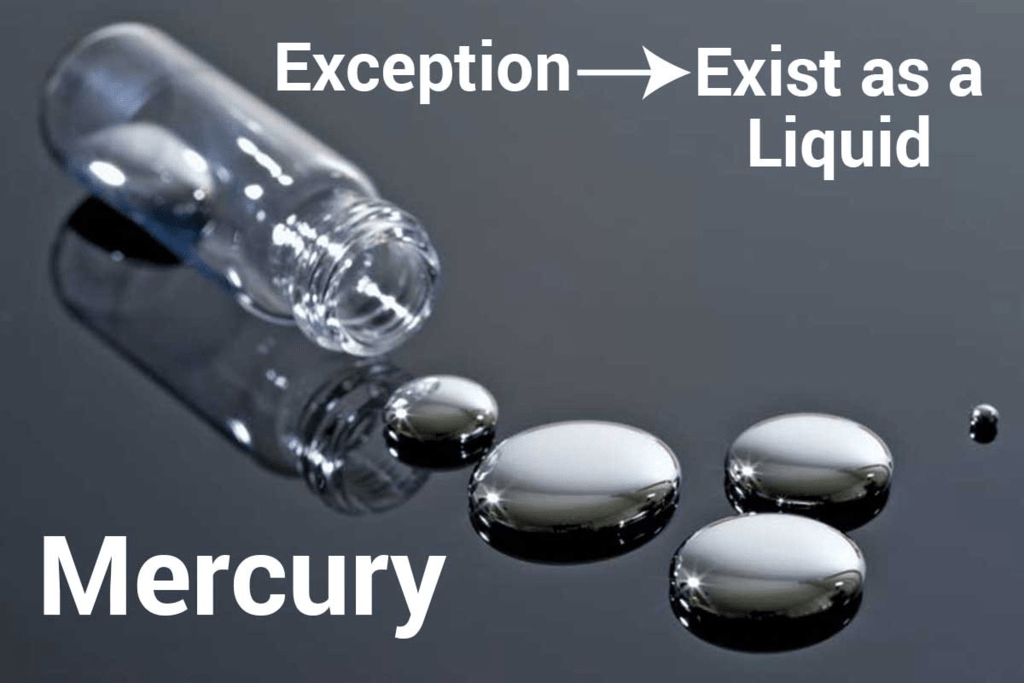
(b) Metal that can be easily cut with a knife is sodium.
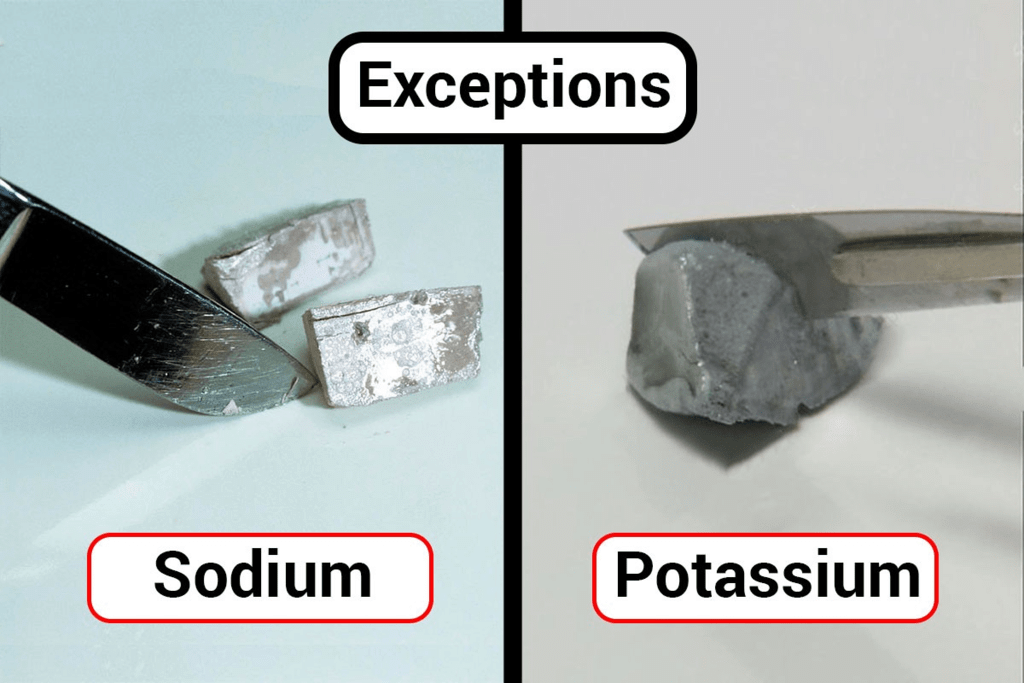 Sodium and Potassium are so soft that they can be cut with a knife.(c) Metal that is the best conductor of heat is silver.
Sodium and Potassium are so soft that they can be cut with a knife.(c) Metal that is the best conductor of heat is silver.
(d) Metal that is a poor conductor of heat is lead.
Q2. Explain the meanings of Malleable and Ductile.
Ans:
Malleable
- Substances that can be beaten into thin sheets are called malleable. Most of the metals are malleable. The most malleable metals are gold and silver.

Ductile
- Substances that can be drawn into thin wires are called ductile. Most of the metals are ductile. Platinum, gold, and silver are the most ductile metals.
 Ductility
Ductility
Page No. 46
Q1. Why is sodium kept immersed in kerosene oil?
Ans:
- Sodium is a very reactive metal and combines explosively with air(oxygen) at room temperature.
- It also reacts violently with cold water. Hence, it catches fire if kept in open.
- Therefore, to prevent accidental fires and accidents, sodium is stored immersed in kerosene oil.
Q2. Write equations for the reactions of
(a) iron with steam
(b) calcium and potassium with water
Ans:
(a) Iron reacts with steam to form a magnetic oxide of Fe with the liberation of H2.
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
(b) Calcium reacts with water to form calcium hydroxide and hydrogen.
Ca(s) + 2H2O(I) → Ca(OH)2(aq) + H2(g)
Potassium reacts with cold water violently immediately with evolution of H2 which catches fire.
2K(s) + 2H2O(I) → 2KOH(aq) + 2H2(g)
Q3. Samples of four metals A, B, C, and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.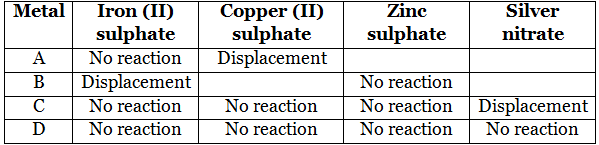
Use the table above to answer the following questions about metals A, B, C, and D.
(a) Which is the most reactive metal?
(b) What would you observe if B is added to a solution of copper (II) sulfate?
(c) Arrange the metals A, B, C, and D in the order of decreasing reactivity.
Ans:
- A + FeSO4 → No reaction, i.e., A is less reactive than iron.
- A + CuSO4 → Displacement, i.e., A, is more reactive than copper.
- B + FeSO4 → Displacement, i.e., B is more reactive than iron.
- B + ZnSO4 →No reaction, i.e., B is less reactive than zinc.
- C + FeSO4 → No reaction, i.e., C is less reactive than iron.
- C + CuSO4 → No reaction, i.e., C is less reactive than copper.
- C + ZnSO4 → No reaction, i.e., C is less reactive than zinc.
- C + AgNO3 → Displacement, i.e., C is more reactive than silver.
- D + FeSO4/CuSO4/ZnSO4/AgNO3 → No reaction, i.e.
- D is less reactive than iron, copper, zinc, and silver.
- From the above equations, we obtain:
(a) B is the most reactive metal.
(b) If B is added to a solution of copper (II) sulfate, then it would displace copper.
B + CuSO4→ Displacement
(c) The arrangement of the metals in the order of decreasing reactivity is: B > A > C > D
Q4. Which gas is produced when diluted hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H2SO4.
Ans: When dilute hydrochloric acid is added to a reactive metal; hydrogen gas is evolved.
The reaction between iron and H2SO4 is:
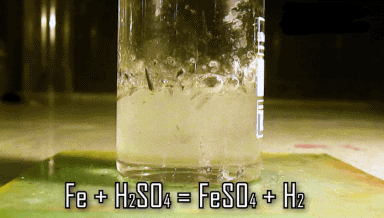
Q5. What would you observe when zinc is added to a solution of iron (II) sulfate? Write the chemical reaction that takes place.
Ans: Zinc is more reactive than iron. Therefore, if zinc is added to a solution of iron (II) sulfate, then it would displace iron from the solution.

Page No. 49
Q1. (a) Write the electron-dot structures for sodium, oxygen, and magnesium.
(b) Show the formation of Na2O and MgO by the transfer of electrons.
(c) What are the ions present in these compounds?
Ans:
(a)
- The electronic configuration of sodium is 2,8,1. The electron dot structure of sodium is
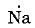
- The electronic configuration of oxygen is 2,6. The electron dot structure of oxygen is
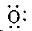
- The electronic configuration of magnesium is 2,8,2. The electron dot structure of magnesium is
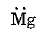
(b) Formation of Na2O and MgO
- Na → Na+ + e-
2,8,1 2,8 - O +2e- → O-
2,6 2,8
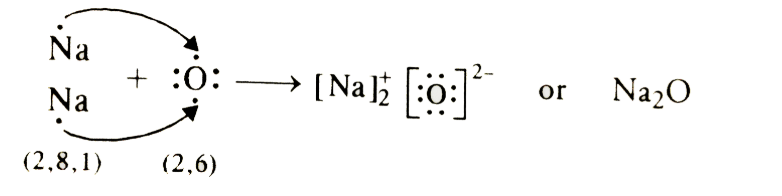
- Mg → Mg2++ 2e-
2,8,2 2,8 - O + 2e- → O2-
2,6 2,8
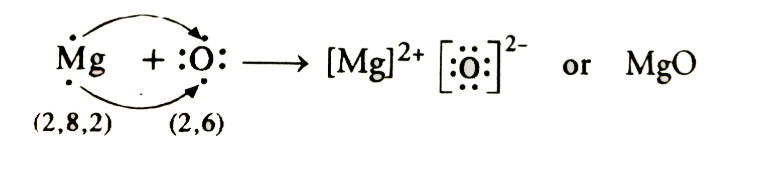
(c) The ions present are Na+, O2- and Mg2+ in compounds sodium oxide (Na2O) and magnesium oxide (MgO).
Q2. Why do ionic compounds have high melting points?
Ans: Ionic compounds have high melting points because of the strong force of attraction between the oppositely charged ions. High energy is required to break the metallic bonds between these ions.
Page No. 53
Q1. Define the following terms.
(a) Mineral
(b) Ore
(c) Gangue
Ans:
(a) Mineral
- The natural materials in which metals occur in the form of their compounds are called minerals.
- They are mostly found in earth’s crust. Some minerals are also found in seawater.
Example: NaCl (sodium chloride), feldspar, mica, kaolin, etc.
(b) Ore
- They are minerals from which metals are extracted profitably.
Example: Hematite (Fe2O3) is an ore of iron, bauxite (Al2O 3.2H2O ) is an ore of aluminium.
(c) Gangue
- The unwanted material present in the ores mined from the earth is called gangue.
- It needs to be removed prior to the extraction process.
Q2. Name two metals which are found in nature in the free state.
Ans: The metals at the bottom of the reactivity series are mostly found in a free state.
Example: Gold, Silver, and Platinum
Q3. What chemical process is used for obtaining a metal from its oxide?
Ans: The chemical process used for obtaining a metal from its oxide is reduction.
There are mainly three different methods of reduction:
(i) By heating
(ii) By using carbon
(iii) By using aluminium, calcium, sodium, etc., as reducing agents.
Page No. 55
Q1. Metallic oxides of zinc, magnesium, and copper were heated with the following metals. In which cases will you find displacement reactions taking place?
In which cases will you find displacement reactions taking place?
Ans: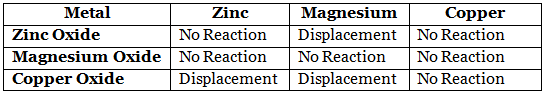
Q2. Which metals do not corrode easily?
Ans: The more reactive a metal is, more likely it is to be corroded. Therefore, less reactive metals are less likely to get corroded. This is why gold plating provides high resistance to corrosion.
Example: Gold, Platinum.
Q3. What are alloys?
Ans: Alloys are homogeneous mixtures of two or more elements. The elements could be two metals or a metal and a non-metal. An alloy is formed by first melting the metal and then dissolving the other elements in it.
Example: Steel is an alloy of iron and carbon.
Excercise (Page 56)
Q1. Which of the following pairs will give displacement reactions?
(a) NaCl solution and copper metal.
(b) MgCl2 solution and aluminium metal.
(c) FeSO4 solution and silver metal.
(d) AgNO3 solution and copper metal.
Ans: This is decided on the basis of the activity series of metal. A metal higher on the activity series can displace a metal lower on the activity series from its salt solution. Thus
(a) No displacement
(b) No displacement
(c) No displacement
(d) Displacement reaction takes place
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq)+ 2Ag(s)
because copper is more reactive than Ag.
Q2. Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) all of the above
Ans: (c)
Explanation:
- Grease and paints are organic matter which can burn on heating.
- So, we do not apply grease or paint on a frying pan to prevent it from rusting. We can prevent it from rusting by applying a coating of zinc.
- Zinc is more reactive than iron, and hence it does not allow the iron to rust.
Q3. An element reacts with oxygen to give a compound a high melting point. This compound is also soluble in water. The element is likely to be
(a) Calcium
(b) Carbon
(c) Silicon
(d) Iron
Ans: (a)
Explanation: Calcium oxide has a high melting point as it is ionic in nature and is soluble in water.
Q4. Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin.
(b) zinc has a higher melting point than tin.
(c) zinc is more reactive than tin.
(d) zinc is less reactive than tin.
Ans: (c) Zinc is more reactive than tin; that is why tin is used.
Explanation: Food cans are coated with tin and not with zinc because zinc is above the tin in the reactivity series means more reactive than tin and can react with food elements preserved in it.
Q5. You are given a hammer, a battery, a bulb, wires, and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Ans: (a)
- Take the sample of metal. Hammer it for a long time. Observe the metal after some time.
- Take the sample of non-metal and hammer it a little. You will observe that metal changes into sheets on hammering, i.e., it is malleable, whereas non-metal is brittle, and it breaks on hammering.
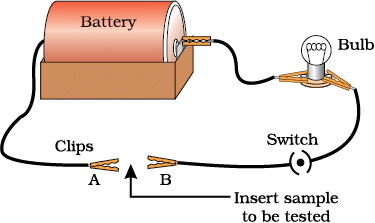
- Set the apparatus, as shown in the figure above.
- Take the sample of metal and put it between the clips. Switch on the current and observe the bulb.
- Now take the sample of non-metal and insert it between clips. Switch on the current and observe the bulb.
- You will observe that the bulb glows when current is switched on in the case of metal sample.
- The bulb does not glow in case of non-metal sample.
- This shows metals are good conductors of electricity, whereas non-metals are bad conductors of electricity.
(b)
- These two tests can be used to distinguish between metals and non-metals.
- Hammering can be used in most metals except in the case of sodium, potassium, and lithium.
- Conduction of electricity can be used in the classification of most of the metals and non-metals except in graphite, which is a non-metallic conductor.
Q6. What are amphoteric oxides? Give two examples of amphoteric oxides.
Ans:
- The oxides which act as both acidic as well as basic are called amphoteric oxides.
Example: Al2O3 and ZnO are amphoteric oxides
Q7. Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Ans: Zn and Al will displace hydrogen from dilute acids because they are more reactive than hydrogen, whereas Cu and Ag cannot displace hydrogen from dilute acids because they are less reactive than hydrogen.
Zn(s) + 2HCl(dil) → ZnCl2(aq) + H2(g)
2Al(s) + 6HCl(dil) → 2AlCl3(aq) + 3H2(g)
Page No. 57
Q8. In the electrolytic refining of a metal M, what would you take as the anode, the cathode, and the electrolyte?
Ans: Impure metal acts as an anode, and pure metal acts as a cathode. Soluble salt of metal acts as an electrolyte.
- When current is passed through the electrolyte, the impure metal from the anode is dissolved in the electrolyte and an equal amount of pure metal from the electrolyte is deposited on the cathode.
Q9. Pratyush took a sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in the figure below.
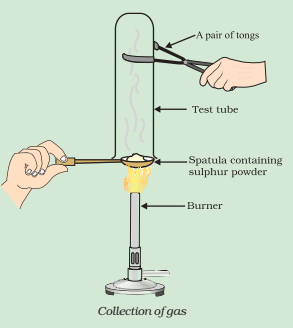
(a) What will be the action of gas on:
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Ans: (a)
(i) There will be no action on dry litmus paper.
(ii) The colour of litmus paper will turn red because sulfur is non-metal, and the oxides of non-metal are acidic in nature.
(b) Chemical Equation for the reaction taking place.
S + O2 → SO2
(Sulphur) Sulphur dioxide
SO2 + H2O → H2SO3
(Sulphurous acid)
Q10. State two ways to prevent the rusting of iron.
Ans: Two ways to prevent the rusting of iron are:
(i) Painting
- Iron articles are painted so that surface does not come in contact with air and water, and it does not get rusted.
(ii) Galvanisation
- It is a process in which iron particles are coated with zinc metal so as to prevent them from rusting.
- Zinc is more reactive than iron; therefore, it loses electrons more readily and prevents iron from rusting.
Q11. What type of oxides are formed when non-metals combine with oxygen?
Ans: Mostly acidic oxides are formed when non-metal combines with oxygen.
Q12. Give reasons.
(a) Platinum, gold, and silver are used to make jewellery.
(b) Sodium, potassium, and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulfide ores are usually converted into oxides during the process of extraction.
Ans:
(a) Platinum, gold, and silver are used to make jewellery because they are:
- Very lustrous.
- Also, they are very less reactive and do not corrode easily.
(b) Sodium, potassium, and lithium are:
- Very reactive metals and react very vigorously with air as well as water.
- Therefore, they are kept immersed in kerosene oil in order to prevent their contact with air and moisture.
(c) Though aluminium is a highly reactive metal, it is resistant to corrosion.
This is because:
- Aluminium reacts with oxygen present in the air to form a thin layer of aluminium oxide.
- This oxide layer is very stable and prevents further reaction of aluminium with oxygen.
- Also, it is light in weight and a good conductor of heat. Hence, it is used to make cooking utensils.
(d) Carbonate and sulfide ores are usually converted into oxides during the process of extraction because metals can be easily extracted from their oxides rather than from their carbonates and sulfides.
Q13. You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Ans:
- Copper reacts with moist carbon dioxide in the air to form copper carbonate, and as a result, the copper vessel loses its shiny brown surface forming a green layer of copper carbonate.
- The citric acid present in the lemon or tamarind neutralises the basic copper carbonate and dissolves the layer.
- That is why tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface of the copper vessel its characteristic lustre.
Q14. Differentiate between metal and non-metal on the basis of their chemical properties.
Ans: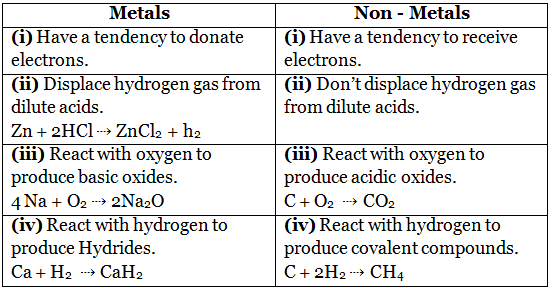
Q15. A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him, which he dipped in a particular solution. The bangles sparkled like new, but their weight was reduced drastically. The lady was upset, but after a futile argument, the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Ans:
- The solution he had used was aqua regia, which is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1.
- Aqua regia is one of the few reagents that are able to dissolve gold.
- When the person claimed to be a goldsmith dipped bangles in aqua regia, some of the gold got dissolved, and hence the weight of the bangles got reduced.
Q16. Give reasons why copper is used to making hot water tanks and not steel (an alloy of iron).
Ans: Copper is used for making hot water tanks because it has excellent thermal conductivity, allowing efficient heat transfer. It is also highly resistant to corrosion, unlike steel, which rusts easily when exposed to water.
|
80 videos|569 docs|80 tests
|
FAQs on NCERT Solutions for Class 10 Science Chapter 3 - Metals and Non-metals
| 1. What are the main differences between metals and non-metals? |  |
| 2. Why are metals considered to be good conductors of electricity? |  |
| 3. What are some common uses of non-metals in everyday life? |  |
| 4. How do metals react with acids? |  |
| 5. What are some physical properties that differentiate metals from non-metals? |  |





















