Nomenclature of Organic Compounds having Functional Groups | Chemistry Class 11 - NEET PDF Download
A functional group, as defined earlier, is an atom or a group of atoms bonded together in a unique manner which is usually the site of chemical reactivity in an organic molecule.
- Compounds having the same functional group undergo similar reactions. For example, CH3OH, CH3CH2OH, and (CH3)2CHOH — all having -OH functional group liberate hydrogen on reaction with sodium metal.
- The presence of functional groups enables the systematisation of organic compounds into different classes.
- First of all, the functional group present in the molecule is identified which determines the choice of appropriate suffix.
- The longest chain of carbon atoms containing the functional group is numbered in such a way that the functional group is attached at the carbon atom possessing the lowest possible number in the chain.
- In the case of polyfunctional compounds, one of the functional groups is chosen as the principal functional group and the compound is then named on that basis. The remaining functional groups, which are subordinate functional groups, are named as substituents using the appropriate prefixes.
- The choice of principal functional group is made on the basis of the order of preference.
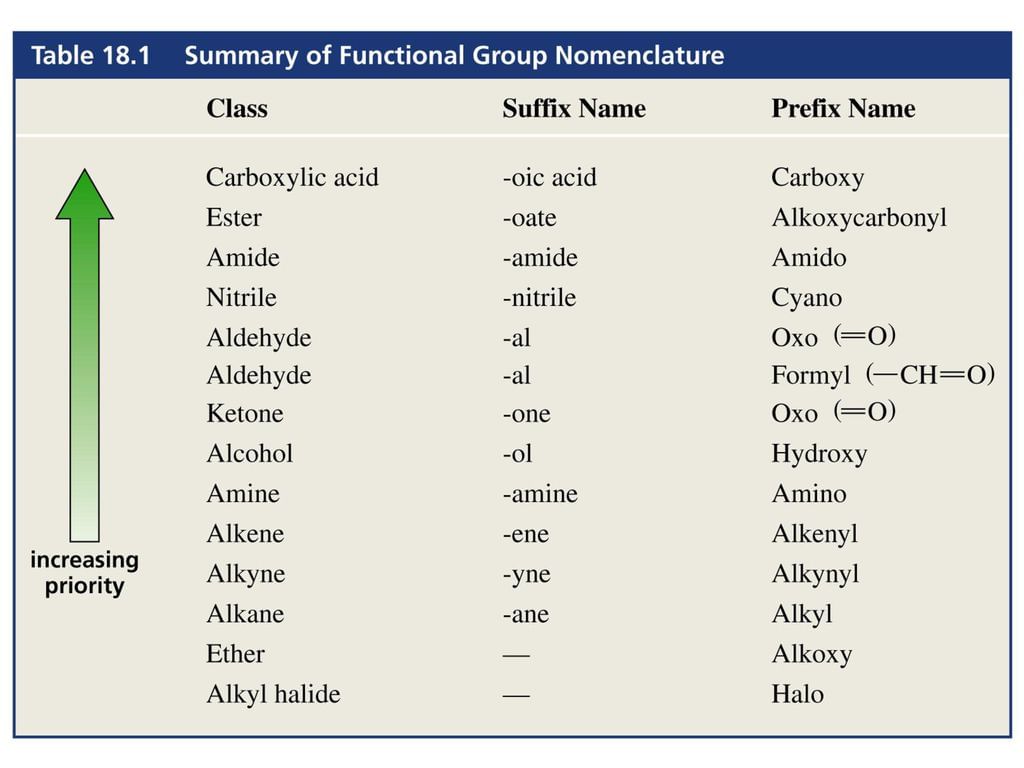
- The –R, C6H5-, halogens (F, Cl, Br, I), –NO2, alkoxy (–OR) etc. are always prefix substituents. Thus, a compound containing both an alcohol and a keto group is named as hydroxyalkanone since the keto group is preferred to the hydroxyl group.
- For example, HOCH2(CH2)3CH2COCH3 will be named as 7-hydroxyheptan-2-one and not as 2-oxoheptan -7-ol. Similarly, BrCH2CH=CH2 is named as 3-bromoprop-1-ene and not 1-bromoprop-2-ene.
- If more than one functional group of the same type are present, their number is indicated by adding di, tri, etc. before the class suffix. In such cases the full name of the parent alkane is written before the class suffix.
- For example, CH2(OH)CH2(OH) is named as ethane–1,2–diol. However, the ending – ne of the parent alkane is dropped in the case of compounds having more than one double or triple bond; for example, CH2=CH-CH=CH2 is named as buta–1,3–diene.
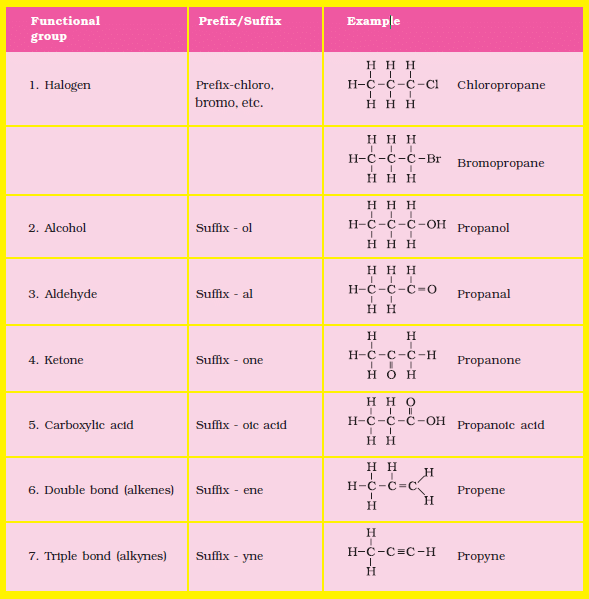
Some Functional Groups and Classes of Organic Compounds
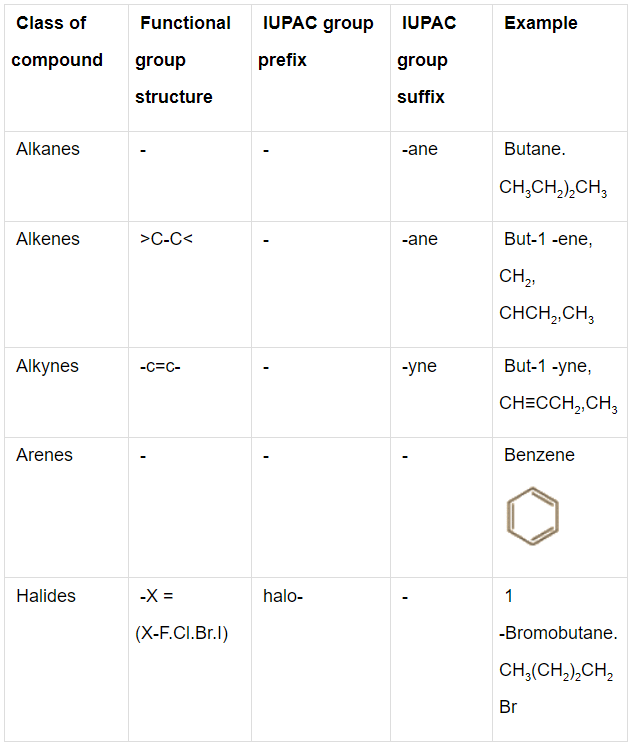
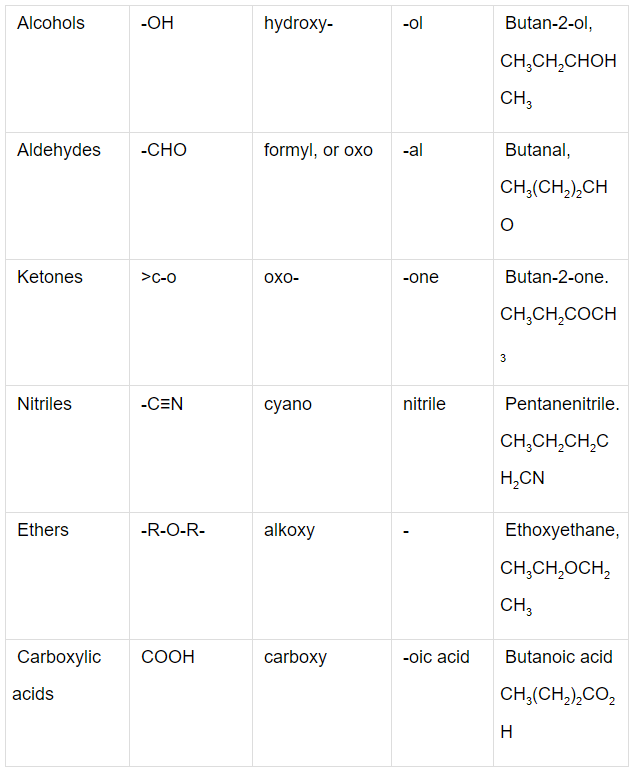
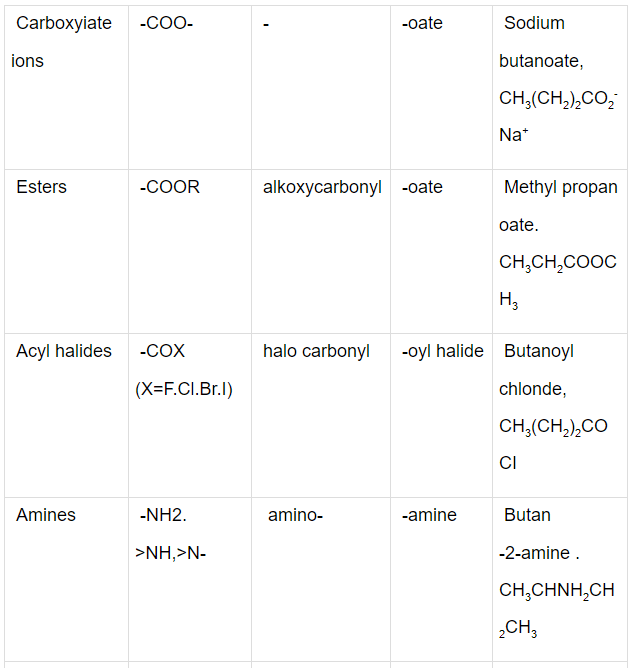
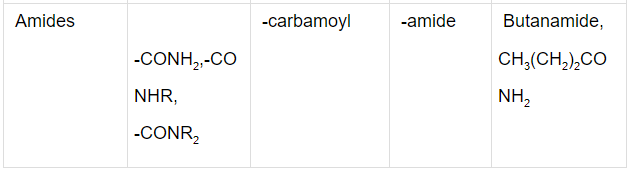
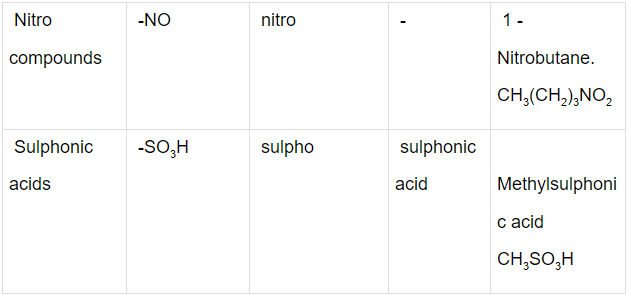
Q.1. Write the IUPAC names of the compounds i-iv from their given structures.
(i) 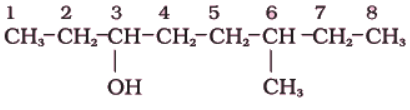
Sol. The functional group present is an alcohol (OH). Hence the suffix is ‘-ol’.
- The longest chain containing -OH has eight carbon atoms. Hence the corresponding saturated hydrocarbon is octane.
- The -OH is on carbon atom 3. In addition, a methyl group is attached at 6th carbon.
Hence, the systematic name of this compound is 6-Methyloctan-3-ol.
(ii) 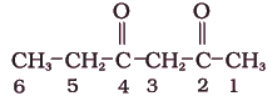
Sol. The functional group present is ketone (>C=O), hence suffix ‘-one’. Presence of two keto groups is indicated by ‘di’, hence suffix becomes ‘dione’. The two keto groups are at carbons 2 and 4. The longest chain contains 6 carbon atoms, hence, parent hydrocarbon is hexane. Thus, the systematic name is Hexane 2,4-dione.
(iii)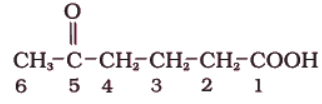
Sol.
Here, two functional groups namely ketone and carboxylic acid are present.
The principal functional group is the carboxylic acid group; hence the parent chain will be suffixed with ‘oic’ acid. Numbering of the chain starts from carbon of – COOH functional group. The keto group in the chain at carbon 5 is indicated by ‘oxo’. The longest chain including the principal functional group has 6 carbon atoms; hence the parent hydrocarbon is hexane. The compound is, therefore, named as 5-Oxohexanoic acid.
(iv) 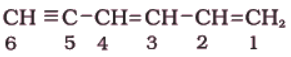
Sol. The two C=C functional groups are present at carbon atoms 1 and 3, while the C≡C functional group is present at carbon 5. These groups are indicated by suffixes ‘diene’ and ‘yne’ respectively. The longest chain containing the functional groups has 6 carbon atoms; hence the parent hydrocarbon is hexane. The name of compound, therefore, is Hexa-1,3-dien-5-yne.
Q. 2. Derive the structure of (i) 2-Chlorohexane, (ii) Pent-4-en-2-ol, (iii) 3- Nitrocyclohexene, (iv) Cyclohex-2-en-1-ol, (v) 6-Hydroxyheptanal.
Sol. (i) ‘hexane’ indicates the presence of 6 carbon atoms in the chain. The functional group chloro is present at carbon 2. Hence, the structure of the compound is CH3CH2CH2CH2CH(Cl)CH3.
(ii) ‘pent’ indicates that parent hydrocarbon contains 5 carbon atoms in the chain. ‘en’ and ‘ol’ correspond to the functional groups C=C and -OH at carbon atoms 4 and 2 respectively. Thus, the structure is
CH2=CHCH2CH (OH)CH3.
(iii) Six membered ring containing a carbon-carbon double bond is implied by cyclohexene, which is numbered as shown in (I). The prefix 3-nitro means that a nitro group is present on C-3. Thus, complete structural formula of the compound is (II). Double bond is suffixed functional group whereas NO2 is prefixed functional group therefore double bond gets preference over –NO2 group:
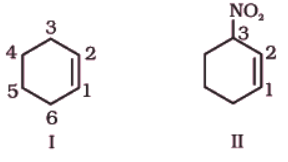
(iv) ‘1-ol’ means that a -OH group is present at C-1. OH is suffixed functional group and gets preference over C=C bond. Thus the structure is as shown in (II):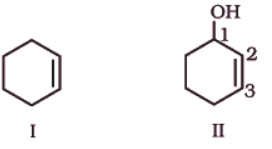
(v) ‘heptanal’ indicates the compound to be an aldehyde containing 7 carbon atoms in the parent chain. The ‘6-hydroxy’ indicates that -OH group is present at carbon 6. Thus, the structural formula of the compound is: CH3CH(OH)CH2CH2CH2CH2CHO. Carbon atom of –CHO group is included while numbering the carbon chain.
Nomenclature of Substituted Benzene Compounds
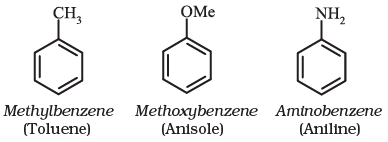
For IUPAC nomenclature of substituted benzene compounds, the substituent is placed as prefix to the word benzene as shown in the following examples. However, common names (written in bracket below) of many substituted benzene compounds are also universally used.
 If benzene ring is disubstituted, the position of substituents is defined by numbering the carbon atoms of the ring such that the substituents are located at the lowest numbers possible. For example, the compound(b) is named as 1,3-dibromobenzene and not as 1,5-dibromobenzene.
If benzene ring is disubstituted, the position of substituents is defined by numbering the carbon atoms of the ring such that the substituents are located at the lowest numbers possible. For example, the compound(b) is named as 1,3-dibromobenzene and not as 1,5-dibromobenzene.
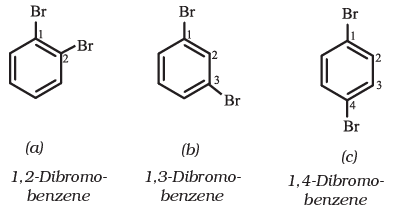 In the trivial system of nomenclature the terms ortho (o), meta (m) and para (p) are used as prefixes to indicate the relative positions 1,2 - ;1,3- and 1,4- respectively.
In the trivial system of nomenclature the terms ortho (o), meta (m) and para (p) are used as prefixes to indicate the relative positions 1,2 - ;1,3- and 1,4- respectively.
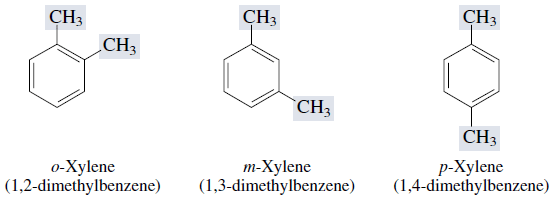
Thus, 1,3-dibromobenzene (b) is named as m-dibromobenzene (meta is abbreviated as m-) and the other isomers of dibromobenzene 1,2-(a) and 1,4-(c), are named as ortho (or just o-) and para (or just p-)-dibromobenzene, respectively.
For tri - or higher substituted benzene derivatives, these prefixes cannot be used and the compounds are named by identifying substituent positions on the ring by following the lowest locant rule. In some cases, common name of benzene derivatives is taken as the base compound.
Substituent of the base compound is assigned number1 and then the direction of numbering is chosen such that the next substituent gets the lowest number. The substituents appear in the name in alphabetical order. Some examples are given below.
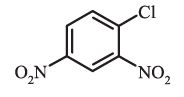 1-Chloro-2,4-dinitrobenzene (not 4-chloro,1,3-dinitrobenzene)
1-Chloro-2,4-dinitrobenzene (not 4-chloro,1,3-dinitrobenzene)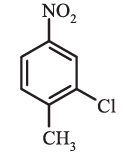 2-Chloro-1-methyl-4-nitrobenzene (not 4-methyl-5-chloro-nitrobenzene)
2-Chloro-1-methyl-4-nitrobenzene (not 4-methyl-5-chloro-nitrobenzene)
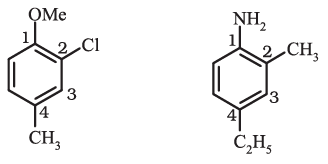 2-Chloro-4-methylanisole 4-Ethyl-2-methylaniline
2-Chloro-4-methylanisole 4-Ethyl-2-methylaniline
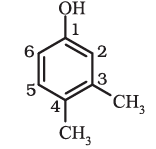 3,4-DimethylphenolWhen a benzene ring is attached to an alkane with a functional group, it is considered as substituent, instead of a parent. The name for benzene as substituent is phenyl (C6H5-, also abbreviated as Ph).
3,4-DimethylphenolWhen a benzene ring is attached to an alkane with a functional group, it is considered as substituent, instead of a parent. The name for benzene as substituent is phenyl (C6H5-, also abbreviated as Ph).
|
114 videos|263 docs|74 tests
|





















