Nucleophilic Addition of Alcohols- Acetal Formation | Chemistry Optional Notes for UPSC PDF Download
Nucleophilic Addition of Alcohols- Acetal Formation
Aldehydes and ketones react reversibly with 2 equivalents of an alcohol in the presence of an acid catalyst to yield acetals, R2C(OR′)2, which are often called ketals if derived from a ketone. Cyclohexanone, for instance, reacts with methanol in the presence of HCl to give the corresponding dimethyl acetal.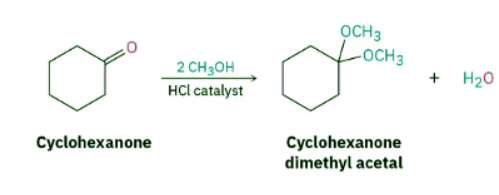
Acetal formation is similar to the hydration reaction. Like water, alcohols are weak nucleophiles that add to aldehydes and ketones slowly under neutral conditions. Under acidic conditions, however, the reactivity of the carbonyl group is increased by protonation, so addition of an alcohol occurs rapidly.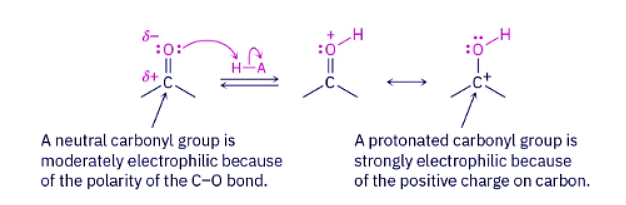
As shown in Figure 19.11, nucleophilic addition of an alcohol to the carbonyl group initially yields a hydroxy ether called a hemiacetal, analogous to the gem diol formed by addition of water. Hemiacetals are formed reversibly, with equilibrium normally favoring the carbonyl compound. In the presence of acid, however, a further reaction occurs. Protonation of the –OH group, followed by an E1-like loss of water, leads to an oxonium ion, R2C═O+R, which undergoes a second nucleophilic addition of alcohol to yield the protonated acetal. Loss of a proton completes the reaction.
Figure 19.11 MECHANISM Mechanism of acid-catalyzed acetal formation by reaction of an aldehyde or ketone with an alcohol.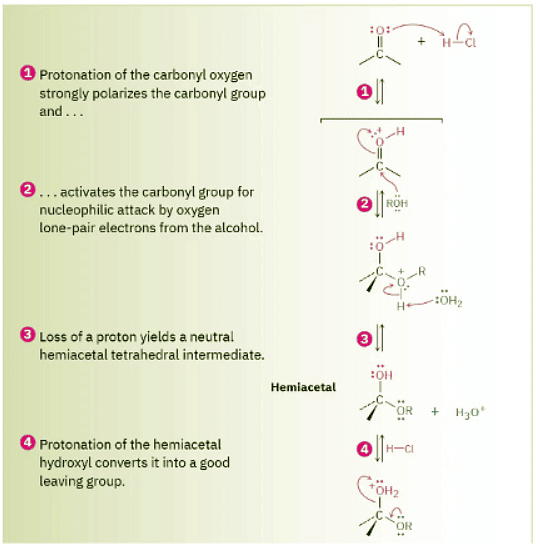
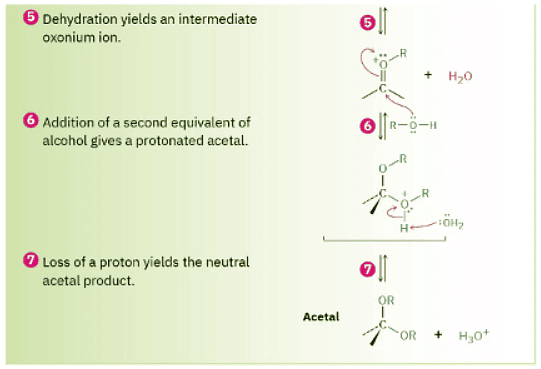
Because all the steps in acetal formation are reversible, the reaction can be driven either forward (from carbonyl compound to acetal) or backward (from acetal to carbonyl compound), depending on the conditions. The forward reaction is favored by conditions that remove water from the medium and thus drive the equilibrium to the right. In practice, this is often done by distilling off water as it forms. The reverse reaction is favored by treating the acetal with a large excess of aqueous acid to drive the equilibrium to the left.
Acetals are useful because they can act as protecting groups for aldehydes and ketones in the same way that trimethylsilyl ethers act as protecting groups for alcohols. As we saw previously, it sometimes happens that one functional group interferes with intended chemistry elsewhere in a complex molecule. For example, if we wanted to reduce only the ester group of ethyl 4-oxopentanoate, the ketone would interfere. Treatment of the starting keto ester with LiAlH4 would reduce both the keto group and the ester group to give a diol product.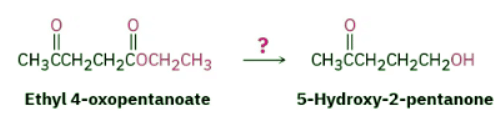
By protecting the keto group as an acetal, however, the problem can be circumvented. Like other ethers, acetals are unreactive to bases, hydride reducing agents, Grignard reagents, and catalytic hydrogenation conditions, but they are cleaved by acid. Thus, we can accomplish the selective reduction of the ester group in ethyl 4-oxopentanoate by first converting the keto group to an acetal, then reducing the ester with LiAlH4, and then removing the acetal by treatment with aqueous acid. (In practice, it’s often convenient to use 1 equivalent of a diol such as ethylene glycol as the alcohol to form a cyclic acetal. The mechanism of cyclic acetal formation using 1 equivalent of ethylene glycol is exactly the same as that using 2 equivalents of methanol or other monoalcohols.)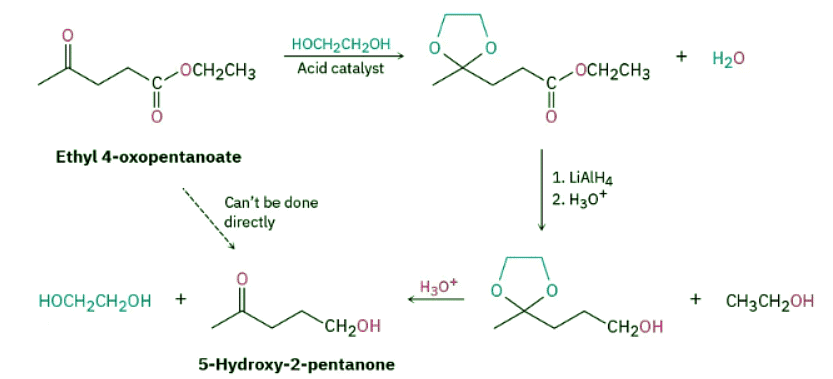
Acetal and hemiacetal groups are particularly common in carbohydrate chemistry. Glucose, for instance, is a polyhydroxy aldehyde that undergoes an internal nucleophilic addition reaction and exists primarily as a cyclic hemiacetal.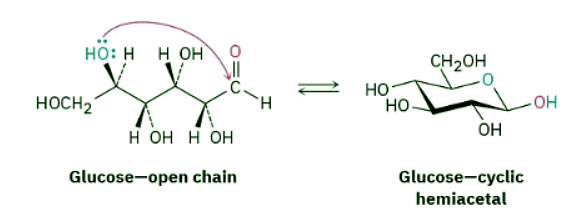
Solved Example
Example: Predicting the Product of Reaction between a Ketone and an Alcohol
Show the structure of the acetal you would obtain by acid-catalyzed reaction of 2-pentanone with 1,3-propanediol.
Ans: Strategy: Acid-catalyzed reaction of an aldehyde or ketone with 2 equivalents of a monoalcohol or 1 equivalent of a diol yields an acetal, in which the carbonyl oxygen atom is replaced by two –OR groups from the alcohol.
Solution: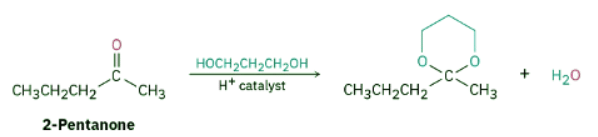
FAQs on Nucleophilic Addition of Alcohols- Acetal Formation - Chemistry Optional Notes for UPSC
| 1. What is nucleophilic addition of alcohols? |  |
| 2. How does acetal formation occur in nucleophilic addition of alcohols? |  |
| 3. What are the applications of acetal compounds formed through nucleophilic addition of alcohols? |  |
| 4. Can all alcohols participate in nucleophilic addition and acetal formation? |  |
| 5. What factors influence the rate of acetal formation in nucleophilic addition reactions? |  |















