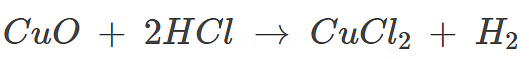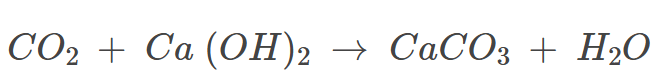Overview: Acids, Bases and Salts | Science Class 10 PDF Download
| Table of contents |

|
| What are Acids and Bases? |

|
| Understanding the Chemical Properties of Acids and Bases |

|
| Acids and Bases in Water |

|
| pH Scale |

|
| More About Salts |

|
What are Acids and Bases?
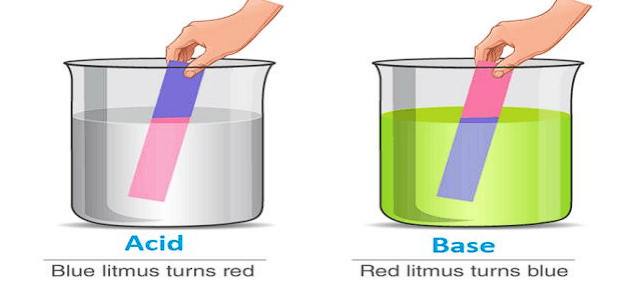
An acid is defined as a substance whose water solution tastes sour, turns blue litmus red and neutralizes bases.
A substance is called base if its aqueous solution tastes bitter, turns red litmus blue or neutralizes acids.
Understanding the Chemical Properties of Acids and Bases
Acids and Bases in the Laboratory
Activity 1
Aim: To identify acidic, basic, and neutral solutions using red litmus paper and observe color changes with different indicators.
Materials Required: Three test tubes (distilled water, acidic solution, basic solution), Red litmus paper, Hydrochloric acid (HCl), Sodium hydroxide (NaOH)
Indicators: Red litmus, Blue litmus, Phenolphthalein, Methyl orange
Procedure:
- Dip red litmus paper into each test tube:
If it turns blue, the solution is basic.
If there’s no change, the solution is either acidic or neutral.
Use the remaining test tubes to distinguish the acidic solution (litmus stays red) from the neutral solution (no color change).- Drop each indicator into small amounts of HCl and NaOH:
- Observe the color changes with red litmus, blue litmus, phenolphthalein, and methyl orange.
Result: Red litmus paper can effectively differentiate between acidic, basic, and neutral solutions. Different indicators provide clear color changes that help confirm the nature of the solution.
Reaction of Acids & Bases with Metals
When a metal reacts with an acid, it generally displaces hydrogen from the acids. This leads to the evolution of hydrogen gas. The metals combine with the remaining part of acids to form a salt.
Activity 2
Aim: To observe the reaction between zinc and dilute sulphuric acid and identify the gas produced.
Materials Required: Test tube, Dilute sulphuric acid (H₂SO₄), Zinc granules, Soap solution, Burning candle
Procedure:
- Add 5 mL of dilute sulphuric acid to a test tube.
- Add a few zinc granules to the acid.
- Observe the reaction on the surface of the zinc granules.
- Pass the gas released through the soap solution and observe the formation of bubbles.
- Bring a burning candle near the gas-filled bubbles and observe the reaction.
Result: Bubbles form in the soap solution due to the gas produced in the reaction between zinc and dilute sulphuric acid. When a burning candle is brought near the bubbles, a pop sound is heard, indicating the presence of hydrogen gas.
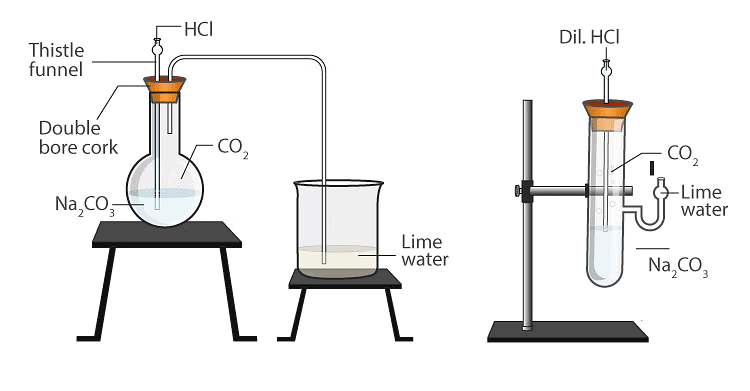
Reaction of Metal Carbonates and Metal Hydrogebcarbonates with Acids
Metal carbonates/metal bicarbonates react with acids to produce salt, carbon dioxide and water.
Activity 3
Aim: To observe the reaction between sodium carbonate (Na₂CO₃) or sodium bicarbonate (NaHCO₃) and hydrochloric acid (HCl) and identify the gas produced.
Materials Required: Test tube, Sodium carbonate (Na₂CO₃) or sodium bicarbonate (NaHCO₃), Hydrochloric acid (HCl), Limewater (Ca(OH)₂ solution), Dropper
Procedure:
- Add a small amount of sodium carbonate or sodium bicarbonate to a test tube.
- Using a dropper, add a few drops of hydrochloric acid to the test tube.
- Observe the effervescence that occurs as the reaction takes place.
- Carefully pass the gas produced through limewater.
- Observe any changes in the limewater.
Result: When sodium carbonate or sodium bicarbonate reacts with hydrochloric acid, carbon dioxide gas (CO₂) is produced, causing effervescence. Passing this gas through limewater results in a milky appearance, confirming the presence of carbon dioxide.
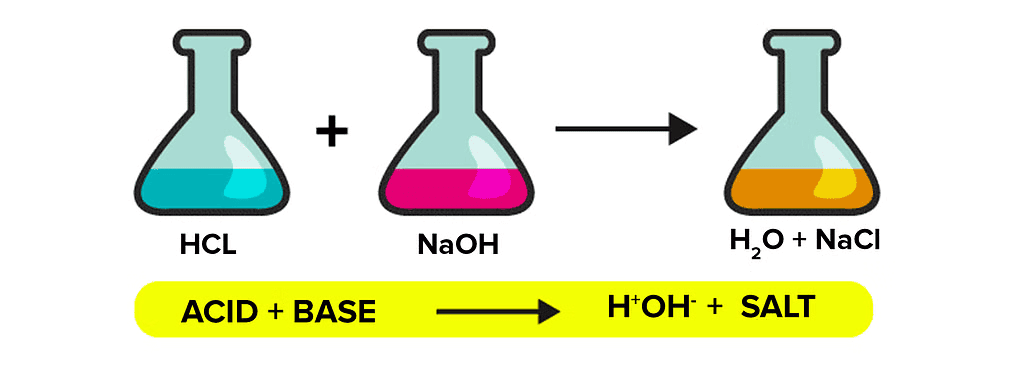
How do Acids and Bases React with each other?
Acids react with bases to produce salt and water. The reaction between acids and bases to give salts is known as neutralization reactions.
Activity 4
Aim: To observe the neutralization reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) and understand the formation of salt and water.
Materials Required: Test tube, Sodium hydroxide (NaOH) solution, Hydrochloric acid (HCl) solution, Phenolphthalein solution and Dropper
Procedure:
- Pour about 2 mL of sodium hydroxide (NaOH) solution into a test tube.
- Add two drops of phenolphthalein solution to the test tube. Observe and note the color of the solution.
- Slowly add hydrochloric acid (HCl) solution drop by drop to the test tube. Observe any color change in the solution.
- Continue adding HCl until the color of the solution changes completely.
- Now, add a few more drops of NaOH solution to the test tube and observe if the original color reappears.
Result:
When phenolphthalein is added to the sodium hydroxide solution, it turns pink, indicating a basic solution. As hydrochloric acid is added, the pink color fades and eventually disappears, signifying the neutralization of the base by the acid. This reaction produces salt (sodium chloride) and water. Adding NaOH again restores the pink color, showing the solution has returned to a basic state. This activity illustrates how an acid and a base react to form a neutral solution, resulting in the production of salt and water.
Reaction of Metallic Oxides with Acids
Acids react with metallic oxides to form salt and water.
Activity 5
Aim: To observe the reaction between copper oxide (CuO) and dilute hydrochloric acid (HCl) and understand the formation of a salt and water.
Materials Required: Beaker, Copper oxide (CuO), Dilute hydrochloric acid (HCl) and Stirring rod
Procedure:
- Place a small amount of copper oxide in a beaker.
- Slowly add dilute hydrochloric acid to the beaker while continuously stirring the mixture.
- Observe and note the color of the solution formed during the reaction.
Result:
As dilute hydrochloric acid is added to copper oxide, the black copper oxide gradually dissolves, forming a blue-green solution of copper chloride (CuCl₂). This indicates that a chemical reaction has occurred, resulting in the formation of a salt (copper chloride) and water. The change in color from black to blue-green signifies the conversion of copper oxide into a soluble salt.
Example: Copper oxide (II), a black metal oxide reacts with dilute hydrochloric acid to form a blue-green coloured copper chloride (II) solution.
Reaction of a Non-metallic Oxide with Base
Non-metal oxides react with bases to produce salt and water.
Example: Calcium hydroxide reacts with non-metallic oxides like carbon dioxide to form calcium carbonate salt and water.
Acids and Bases in Water
Acids in Water
- When an acid is dissolved in water, it furnishes hydrogen ions, and consequently, the concentration of hydrogen ions (H+) increases in the solution.
- The reaction is highly exothermic in nature due to the production of heat.
Activity 6
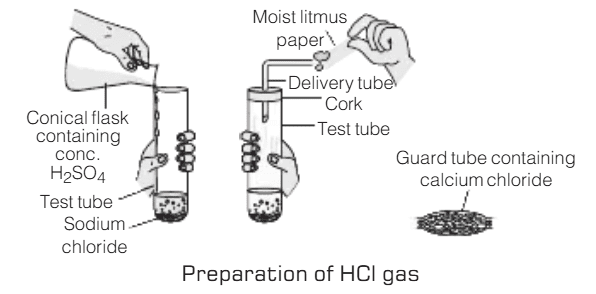
Aim: To study the effect of dry and wet blue litmus paper on: i) Dry HCl gas ii) HCl solution
Materials Required: Test tubes, Sodium chloride (NaCl) salt, Concentrated sulfuric acid (H₂SO₄), Dry and wet blue litmus paper strips, Delivery tube and Cork
Procedure:
- Take about 1 gram of solid NaCl in a clean and dry test tube.
- Add a small amount of concentrated H₂SO₄ to the test tube.
- A gas (dry HCl gas) will be evolved.
- Test the gas with both dry and wet blue litmus paper strips.
- Record the color change observed on the litmus paper.
Observations:
- Dry HCl Gas:
The wet (moist) blue litmus paper turns red upon exposure to the gas.
No color change is observed on the dry blue litmus paper.- HCl Solution:
The color of both wet and dry blue litmus papers turns red when exposed to HCl solution.Result:
The experiment shows that dry HCl gas does not affect dry blue litmus paper but turns wet blue litmus paper red. This indicates that hydrogen ions (H⁺) responsible for the acidity of HCl are only produced in the presence of water, leading to a color change in the wet litmus paper. When HCl is in solution, both dry and wet blue litmus papers turn red, confirming the presence of H⁺ ions in the solution.
Bases in Water
- When a base is dissolved in water, it produces hydroxide ions (OH-).
- The reaction can be represented as: NaOH (aq) → Na+ (aq) + OH- (aq).
pH Scale
pH of a solution: pH of a solution is the negative logarithm to the base 10 of the hydrogen ion concentration expressed in mole per litre.
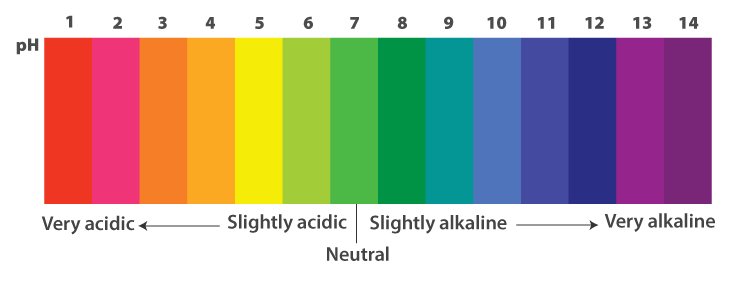
Importance of pH in everyday life
pH change and survival of animals
- Our body works well within a narrow pH range of 7.0 to 7.8.
- When the pH of rain water is less than 5.6, it is known as acid rain.
- When this acid rain flows into rivers, it lowers the pH of the river water making the
- survival of aquatic life difficult.
pH in our digestive system
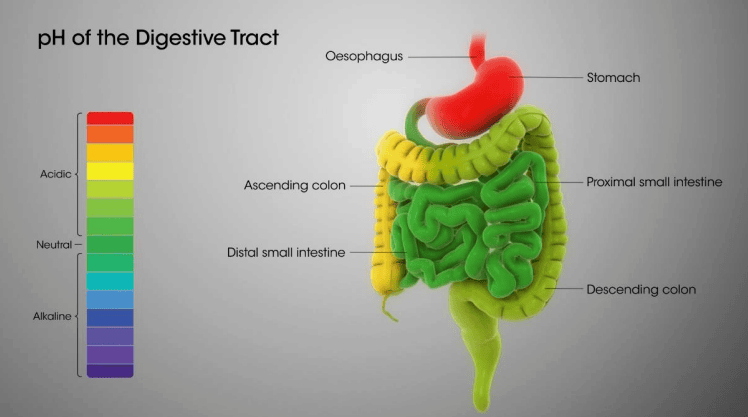
- Our stomach produces hydrochloric acid which helps in the digestion of food without harming the stomach.
- Sometimes excess acid is produced in the stomach which causes indigestion.
- To get rid of this pain, bases called antacids are used.
pH change - Cause of tooth decay
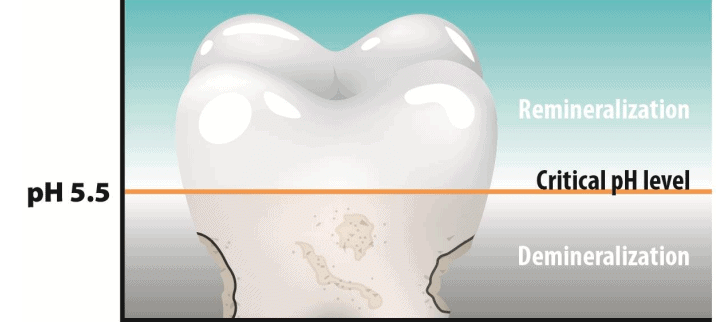
Tooth decay starts when the pH in the mouth falls below 5.5
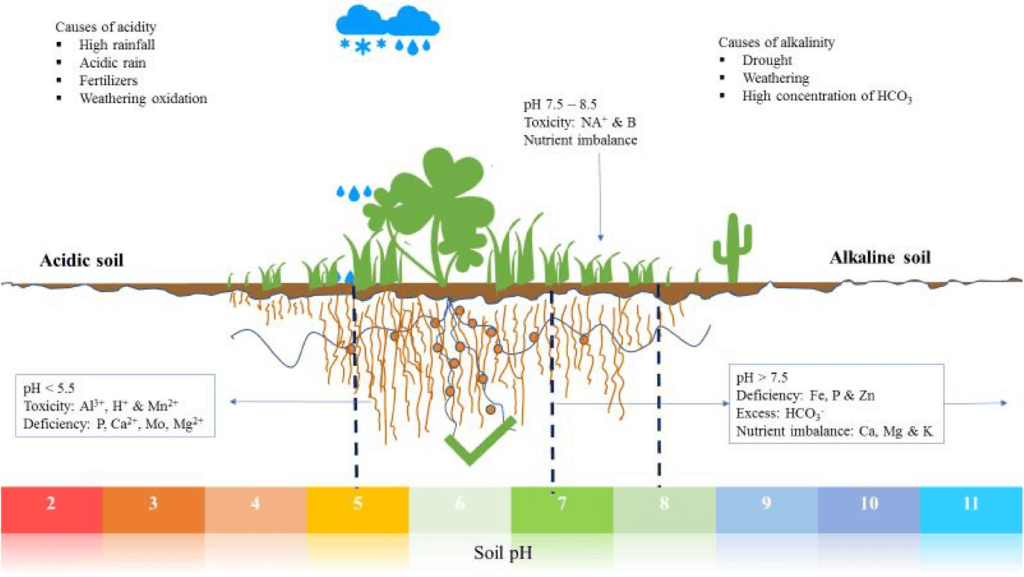
Soil of pH and plant growth
Activity 7
Aim: To determine the pH of soil in your region and assess its suitability for plant growth.
Materials Required: Test tube, Soil sample (about 2 g), Water (5 mL), Filter paper, Funnel, Universal indicator paper and pH chart
Procedure:
- Place about 2 g of soil in a clean test tube.
- Add 5 mL of water to the soil in the test tube.
- Shake the test tube thoroughly to mix the soil and water.
- Set up a funnel with filter paper, and filter the contents of the test tube.
- Collect the clear filtrate in another clean test tube.
- Dip a strip of universal indicator paper into the filtrate.
- Compare the color change on the indicator paper with the pH chart provided.
Observation: By comparing the color change on the universal indicator paper with the pH chart, you can determine the pH of the soil sample. The ideal soil pH for plant growth typically ranges from 6 to 7.5, depending on the specific plants being grown.
Result:
Based on the pH reading, you can conclude whether the soil in your region is suitable for the growth of plants. Soil with a pH within the ideal range supports optimal nutrient availability for plants. If the pH is too low (acidic) or too high (alkaline), it may affect plant growth, and soil amendments may be necessary to adjust the pH for better plant health.
More About Salts
A salt is a combination of an anion of an acid and a cation of a base.
Examples: KCl, NaNO3 ,CaSO4, etc.
Family of salts
Salts having same positive ions (or same negative ions) are said to belong to a family of salts. For example, NaCl, KCl, LiCl.
pH of Salts
- Salts of strong acid and a strong base are neutral, with a pH value of 7.
For Example: NaCl, Na2SO4 - Salts of strong acid and weak base are acidic, with a pH value less than 7.
For Example: Ammonium chloride solution has pH value of 6. - Salts of weak acid and strong base are basic, with a pH value more than 7.
For Example: Sodium carbonate solution has a pH value of 9.
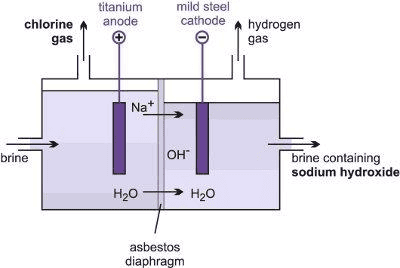
Sodium Hydroxide
- Sodium hydroxide is produced by the electrolysis of an aqueous solution of sodium chloride (called brine).
- The process is called the chlor-alkali process because of the products formed, i.e. ‘chlor’ for chlorine and ‘alkali’ for sodium hydroxide .
Bleaching Powder
- It is produced by the action of chlorine on dry slaked lime [Ca(OH)2]
- Ca(OH)2 + Cl2 → CaOCl2 + H2O
- It is represented as CaOCl2
Baking Soda
- Chemical formula: NaHCO3
- It is produced on a large scale by treating cold and concentrated solution of sodium chloride (brine) with ammonia and carbon dioxide.
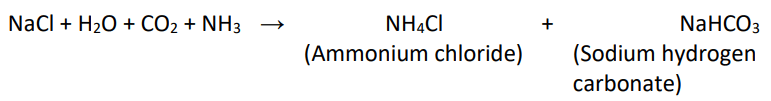
- On heating, it decomposes to give sodium carbonate with the evolution of carbon dioxide.
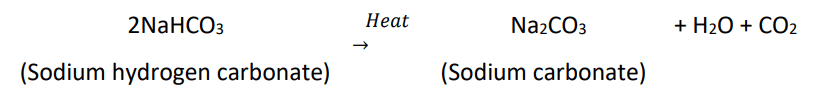
Washing Soda
- Chemical formula: Na2CO3.10H2O
- Sodium hydrogen carbonate, on heating decomposes to give sodium carbonate with the release of hydrogen gas.
- Re-crystallisation of sodium carbonate produces washing soda.
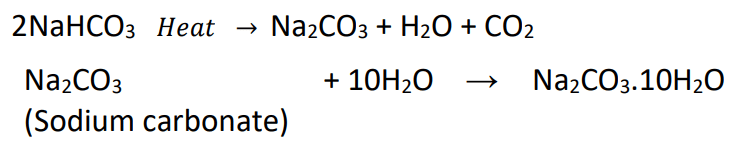
Water Of Crystallisation
Water molecules which form a part of the structure of a crystal are called water of crystallisation. 
Plaster of Paris
- Plaster of Paris is prepared by heating gypsum at 373 K.
- On heating, it loses water molecules and becomes calcium sulphate hemihydrate (CaSO4. 12H2O) which is called Plaster of Paris.
- Uses of Plaster of Paris:
|
80 videos|569 docs|80 tests
|
FAQs on Overview: Acids, Bases and Salts - Science Class 10
| 1. What are some common examples of acids and bases? |  |
| 2. How do acids and bases react in water? |  |
| 3. What is the pH scale and how is it related to acids and bases? |  |
| 4. How can the strength of an acid or base be determined? |  |
| 5. How are salts related to acids and bases? |  |