Overview: Carbon Compounds & Covalent Bonding in Carbon Compounds | Science Class 10 PDF Download
What are Carbon Compounds?
Carbon is one of the most reactive elements in the world. It can form a staggering number of compounds with other elements - so much so that compounds containing carbon outnumber compounds of all other elements combined! Carbon compounds are defined as chemical substances containing carbon.
- Even the word 'carbon' itself is derived from 'carbo', meaning coal.
- Its atomic number is 6, atomic mass is 12, electronic configuration is 2, 4, and valency is 4.
- The compounds obtained from carbon are used in a wide variety of applications - from clothes to medicines, books to food, fertilizers to fuels, and everything in between. In fact, all living structures are carbon-based!
- Despite the relatively low amount of carbon present in the earth's crust and atmosphere - only 0.02% and 0.03% respectively - the importance of this element cannot be overstated.
 Application of Carbon
Application of Carbon - And the applications of carbon are truly limitless. Carbon forms countless compounds with hydrogen, known as hydrocarbons, but can also contain other elements like oxygen, halogens, nitrogen, phosphorus, and sulfur.
- In fact, the number of carbon compounds is so vast that it exceeds three million - which is more than all the compounds formed by any other element combined! So, the next time you see or use something made with carbon, remember the immense role this element plays in our daily lives.
What is Covalent Bond?
A chemical bond formed between two atoms of the same element or two atoms of different elements by sharing of electrons is called a covalent bond.
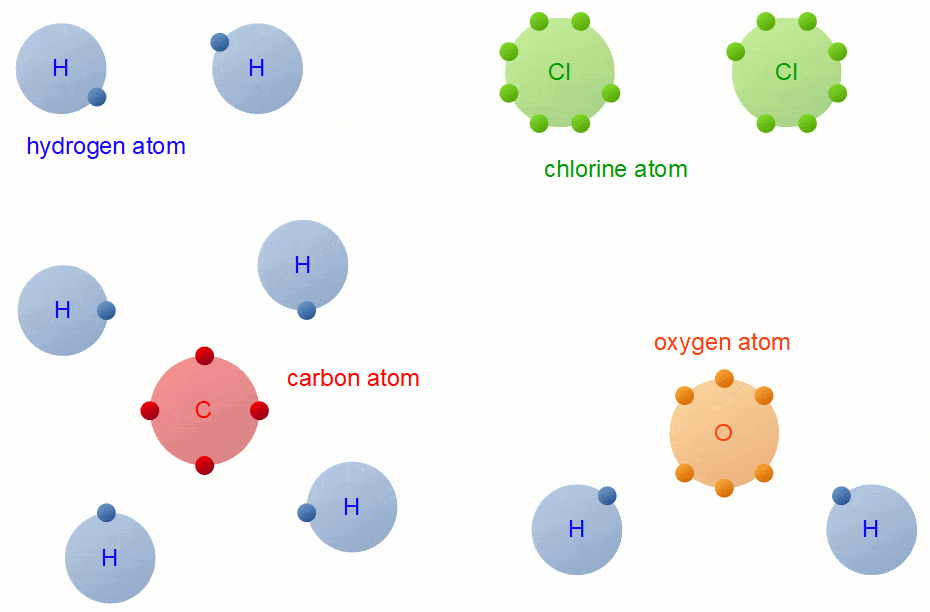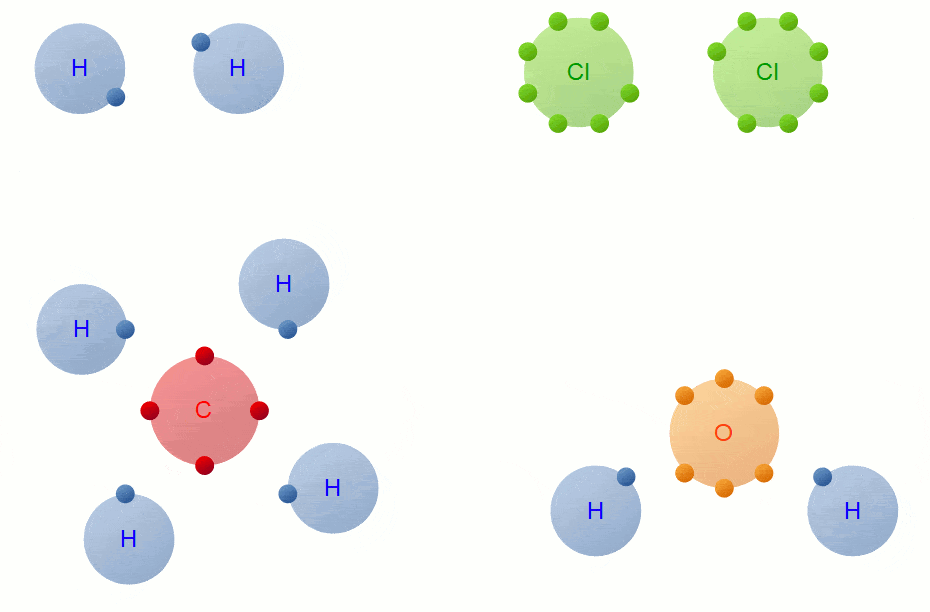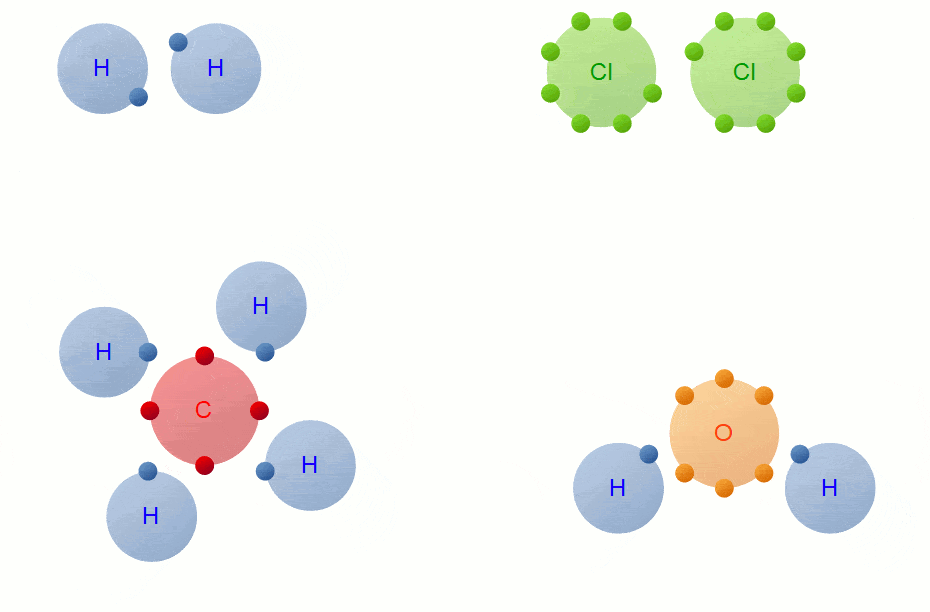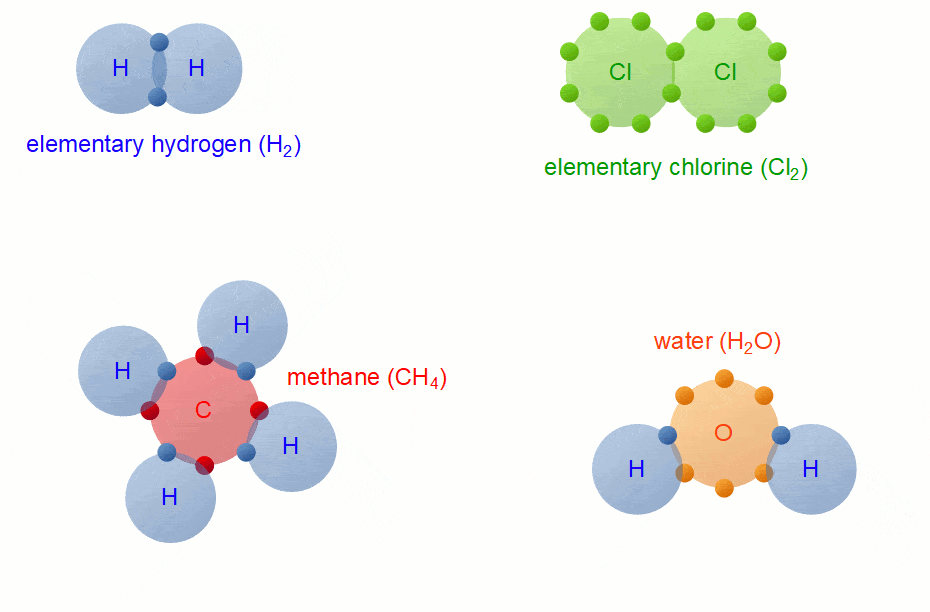
Covalent Bond Formation
Necessary conditions of the formation of Covalent Bond:- The combining atoms should have a nonmetallic character.
- The combining atoms should contain 4 to 7 electrons in their respective valence shell.
- In hydrogen, there is only 1 valence electron, but it also forms a covalent bond.
- The combining atoms need 1, 2, 3 or 4 electrons to complete their octet (hydrogen completes its duplet).
- The combining atoms should contribute an equal number of electrons to form pair of electrons to be shared.
- After sharing the pair of electrons each combining atoms should attain a stable electronic configuration like its nearest noble gas.
Bonding in Carbon Compounds
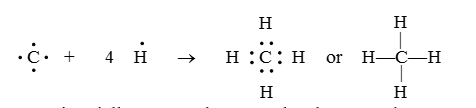
Carbon forms covalent bonds in its compounds with other atoms. In each compound, the valency of carbon is four i.e., carbon has a tetravalent character.Why does a carbon atom form only a covalent bond?
- The atomic number of carbon is 6 and the first shell contains just two electrons and the second shell (Outermost shell) contains four electrons.
- The carbon atom can attain the noble gas configuration by sharing its valence electrons with other atoms of carbon or with atoms of other elements and form a covalent bond.
- Carbon has 4 electrons in its valence shell. To attain stability, it should either gain 4 electrons or lose 4 electrons. It cannot lose 4 electrons as it involves a lot of energy. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the four extra electrons added. Therefore, to complete the octet, it shares 4 electrons with other atoms. That is why, carbon forms compounds mainly by covalent bonding. Question for Overview: Carbon Compounds & Covalent Bonding in Carbon CompoundsTry yourself:Why does carbon form compounds mainly by covalent bonding?View Solution
Characteristics of Covalent Compounds
A covalent bond is formed by the mutual sharing of electrons.Note: Shared pair of electrons is also called a bonding pair of electrons.
- Physical State: The covalent compounds are generally gases or liquids, but compounds with high molecular masses are solids.
Example: Urea, Glucose, Naphthalene (solids); Water, ethanol, benzene (liquids); Methane, chlorine, hydrogen, oxygen (gases). - Melting and boiling points: Covalent compounds have low melting and low boiling points because intermolecular forces (cohesive forces) in covalent compounds are weaker than those in ionic compounds.
Note: Some exception like diamond and graphite which are covalent solids have very high M.P. & B.P.
- Solubility: Covalent compounds generally dissolve readily in organic solvents but they are less soluble in water.
Example: Naphthalene which is an organic compound dissolves readily in organic solvents like ether but is insoluble in water. However, some covalent compounds like urea, glucose, sugar etc. are soluble in water. Some polar covalent compounds like ammonia and hydrochloric acid are soluble in water. - Conductivity: Covalent compounds do not conduct electricity because they contain neither the ions nor free electrons necessary for conduction, So they do not conduct electricity
Example: Covalent compounds like glucose, alcohol, carbon tetrachloride do not conduct electricity.
Classification of Covalent Bond
On the basis of the number of electrons shared by two combining atoms, the covalent bond are of three types:
(a) Single Covalent Bond
- A single covalent bond is formed by the sharing of one pair of electrons between the two atoms. It is represented by one short line (—) between the two atoms.
Example: H-H, Cl -Cl, H-Cl, CH3-CH3.
(b) Double Covalent Bond
- A double covalent bond is formed by the sharing of two pairs of electrons between the two combining atoms. It is represented by putting (=) two short lines between the two bonded atoms.
Examples: O = O (O2), CO2 (O = C = O), H2C = CH2
(c) Triple Covalent Bond
- A triple bond is formed by the sharing of three pair of electrons between the two combining atoms. It is represented by putting three short line (≡) between two bonded atoms.
Example: N2 (N≡N), CH≡CH. N—N Triple Covalent Bond
N—N Triple Covalent Bond
Formation of Covalent Compounds
Hydrogen molecule (H2)
A molecule of hydrogen is composed of two H-atoms.
The electronic configuration of H-atom is:
Shell K: 1 Electron - Incomplete Duplet (Unstable)
It becomes stable when it has 2 electrons in its outer shell and forms a duplet configuration.

H-H Bond in terms of energy shells (orbits)
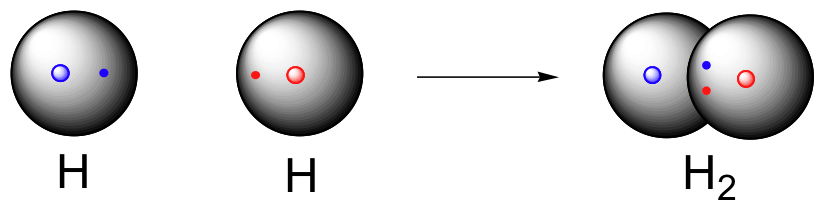 H-H Bond FormationChlorine molecule (Cl2)
H-H Bond FormationChlorine molecule (Cl2)
The atomic number of chlorine is 17, thus there are 17 electrons in an atom of chlorine.
Electronic configuration of Cl atom
Electronic configuration of Ar atom (closest element with stable configuration)
Chlorine atom needs one electron more to complete its octet
Cl-Cl bond in terms of energy shell orbits
 Cl2 molecule formation
Cl2 molecule formation
Hydrochloric acid (HCl)
H atom has one valence electron. It needs 1 electron more to complete its duplet and the chlorine atom has 7 valence electrons. It needs 1 electron more to complete its octet and acquire a stable electronic configuration, (2, 8, 8) like the noble gas argon. Hence they will share an electron to form HCl.

Oxygen molecule(O2)
The atomic number of the O atom is 8. There is 6 electron in the valence shell of an oxygen atom and it needs 2 more electrons to attain the nearest stable inert gas Neon (2, 8) configuration. Hence an oxygen atom will share 2 electrons with other oxygen atom to attain a stable configuration.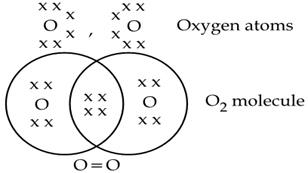
Nitrogen molecule (N2)
The atomic number of nitrogen is 7 and its electronic configuration is K(2), L(5). It needs 3 electrons more to complete its octet like noble gas neon (2, 8). Hence a nitrogen atom will share 3 electrons with other nitrogen atom to attain a stable configuration.
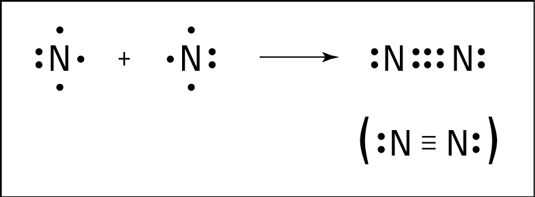 Ammonia molecule (NH3)
Ammonia molecule (NH3)
The atomic number of N is 7. Its electronic configuration is (2, 5), there are 5 electrons in its valence shell. It needs 3 electrons more to complete its octet like noble gas neon (2, 8).
 Ammonia Molecule Formation
Ammonia Molecule Formation H2O molecule
The electronic configuration of hydrogen is K (1) and that of oxygen is K(2) L(6). Thus, each hydrogen atom requires one and the oxygen atom requires two more electrons to achieve the stable electronic configuration, which they will share with each other.
 H2O Molecule Formation
H2O Molecule FormationThe atomic number of Carbon is 6 and the electronic configuration of Carbon is K(2), L(4) and that of oxygen is K(2), L(6) thus each carbon atom requires 4 and the oxygen atom requires two more electrons to achieve the stable electronic configuration. Hence the carbon atom will share its 4 electrons with2 oxygen atoms which will each share 2 of its electrons with the carbon atom to achieve the stable configuration.
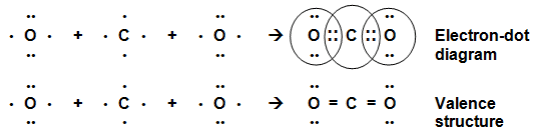 CO2 Molecule Formation
CO2 Molecule FormationCH4 molecule
Methane is a covalent compound containing 4 covalent bonds. It contains one carbon atom and four hydrogen atoms covalently bonded to the central carbon atom.
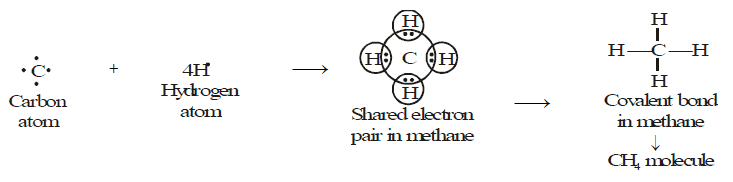 CH4 Bond Formation
CH4 Bond Formation
The electronic configuration of carbon and chlorine atoms are (2, 4) and (2, 8, 7) respectively. The carbon atom needs four electrons and the chlorine atom needs one electron more to attain the stable electronic configuration.
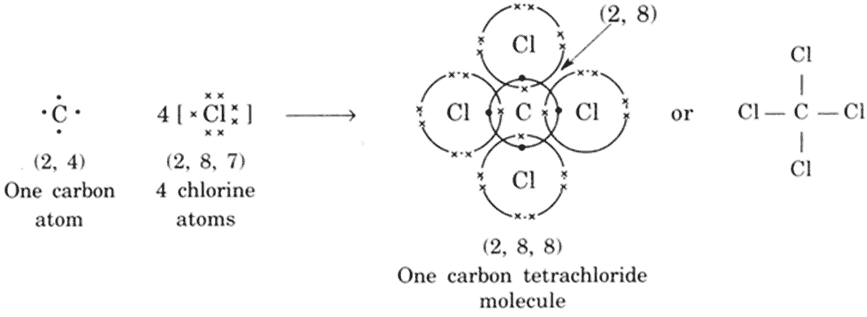 CCl4 Molecule FormationEthene molecule (C2H4)
CCl4 Molecule FormationEthene molecule (C2H4)The electronic configuration of the carbon atom is 2, 4. There are 4 valence electrons in one C atom. Each H atom contains 1 valence electron. Thus, there are 12 valence electrons present in the ethene molecule.
 Ethene Molecule Formation
Ethene Molecule FormationA carbon atom forms a triple covalent bond with another carbon atom and a single covalent bond with a hydrogen atom.
 Ethyne molecule formation
Ethyne molecule formationNon-Polar & Polar Covalent Compounds
- Non-polar covalent bond
A covalent bond formed between two atoms of the same element or the same electronegativity is called a nonpolar covalent bond.
Example: H2, N2, O2, Cl2 etc. - Polar covalent bond
The covalent bond formed between the atoms of two elements having different electronegativities is called a polar covalent bond. The molecules in which the atoms are bonded by a polar covalent bond are called polar molecules.
Note: In a polar covalent bond, the shared pair of electrons lies more toward the atom which is more electronegative.
Example: HCl, H2O & NH3
Table: Differences between ionic and covalent compounds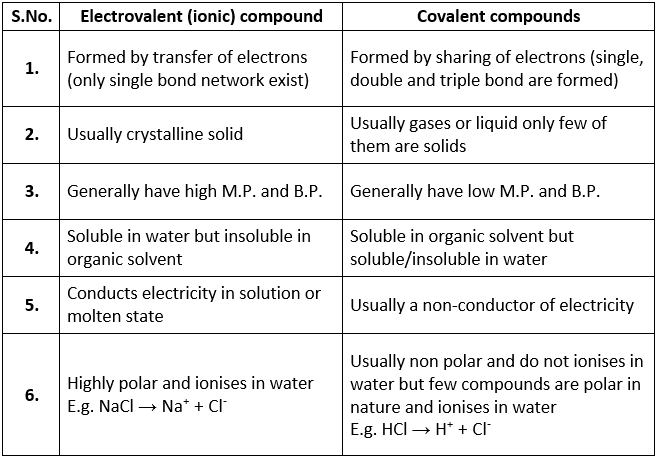
Organic Compounds
The chemical compounds which are present in living organisms (plant and animal) are called organic compounds. - The belief that the formation of organic compounds was possible only in plants and animals led the scientists of early days to propose that the Vital Force was necessary for the formation of such compounds.
- But the experimental work of Friedrich Wohler (German chemist) denied the idea of vital force when he prepared urea in his laboratory. (urea is an organic compound and waste product of urine).
Types of Carbon Compounds
1. Saturated Carbon Compounds
These are the compounds in which various carbon atoms in a chain or a ring are linked together by single bonds only. Alkanes are the most common examples of saturated chain carbon compounds. Ethane is a member of the alkane family whose structure is drawn below: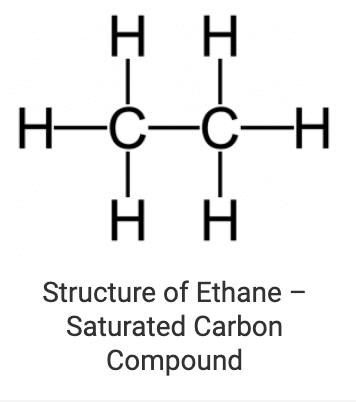
2. Unsaturated Carbon Compounds
These are the compounds in which various carbon atoms in a chain or a ring are linked together by double or triple bonds. Alkenes (where carbon atoms are linked through double bonds) and alkynes (where carbon atoms are linked through triple bonds) are the most common examples of unsaturated chain carbon compounds. Ethene is a member of the alkene family whose structure is drawn below: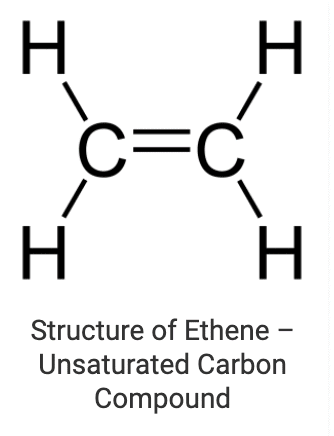
Versatile Nature of Carbon
About three million (or thirty lakh) compounds of carbon are known. The existence of such a large number of organic compounds is due to the following characteristic features of carbon.(a) Catenation
(Tendency to form Carbon-Carbon bond)Carbon has a unique feature of forming long carbon chains i.e. it attaches with other carbon atoms to form long carbon chains. This property is known as catenation.
- The self linking ability of carbon to combine with other atoms of carbon to form long-chain, branched-chain and closed ring structures is called catenation.
- Carbon has the maximum tendency for catenation in the periodic table. This is because of strong carbon-carbon bonds as compared to other atoms and their small size.
- When two or more carbon atoms combine with one another, they form different types of the chain such as:
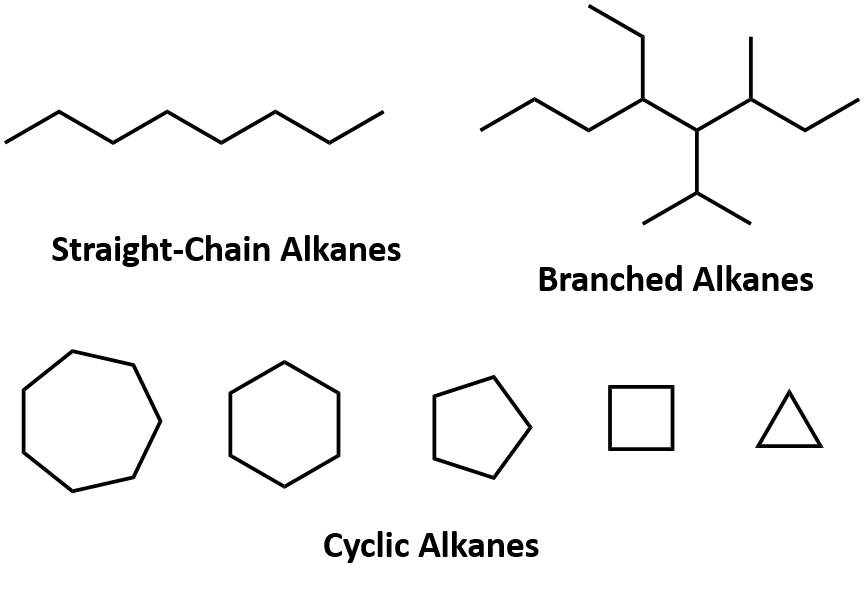
(i) Straight chains
(ii) Branched chains
(iii) Closed chain or ring chain Types of the Carbon Chain
Types of the Carbon Chain
(b) Tetravalency of Carbon
- The atomic number of carbon is 6.
- The electronic configuration of the carbon atom is 2, 4.
- It has four electrons in the outermost shell, therefore its valency is four. Thus, carbon forms four covalent bonds in its compounds.
 Formation of Methane Molecule
Formation of Methane Molecule
(c) Tendency to form Multiple Bonds
Due to its small size, carbon can easily form double or triple bonds (called multiple bonds) with itself and with the atoms of other elements such as nitrogen, oxygen, sulphur etc.(d) Isomerism
Compounds having the same molecular formula but different structural formulae are known as isomers and the phenomenon of the existence of isomers is termed isomerism.
(i) Are good conductors of electricity.
(ii) Are poor conductors of electricity.
(iii) Have strong forces of attraction between their molecules.
(iv) Do not have strong forces of attraction between their molecules.
Chemical Properties of Carbon Compounds
The various chemical properties of carbon are as follows:–1) Atomic Number: The atomic number of carbons is 6.
2) Atomic Mass: Its atomic mass is 12.011 g mol-1.
3) Electronegativity: According to Pauling, the electronegativity of carbon is 2.5.
4) Boiling Point: Its boiling point is 4827o C.
5) Melting Point: Its melting point is 3652o C.
6) Density: Its density is 2.2 g cm-3 at 20o C.
7) Ionic Radius: The ionic radius for C4- is 0.26 nm and for C4+ is 0.015 nm.
8) Van Der Waals Radius: Its Van Der Waals radius is 0.091 nm.
9) Isotopes: It has three isotopes.
10) Electronic Shell: The electron shell configuration of carbon is [He]2s22p2.
11) Energy of first ionisation: The energy of first ionisation of carbon is 1086.1 KJ mol-1.
12) Energy of second ionisation: The energy of the second ionisation of carbon is 2351.9 KJ mol-1.
13) Energy of third ionisation: The energy of the third ionisation of carbon is 4618.8 KJ mol-1.
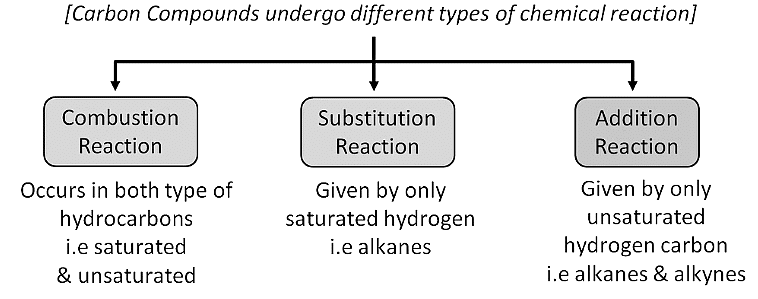
Combustion
- It is the process of burning carbon compounds in the presence of oxygen ( air) to release CO2 and energy in the form of heat and light.
- Combustion is also known as burning.
- Alkanes are very good fuels because when they burn in air, they produce a lot of energy. For example, methane CH4 is a component of natural gas .
- When methane burns in abundance of oxygen (sufficient supply of air), complete combustion takes place to produce CO2 , H2O and energy.
- The saturated hydrocarbons usually burn in air with a blue, non-sooty flame i.e. complete combustion takes place. But if they are burnt in limited oxygen supply, incomplete combustion takes place (a yellow and sooty flame is produced).
- On the other hand, unsaturated hydrocarbons burn in air to produce a yellow sooty flame . However, if they are burnt in pure oxygen, complete combustion takes place.
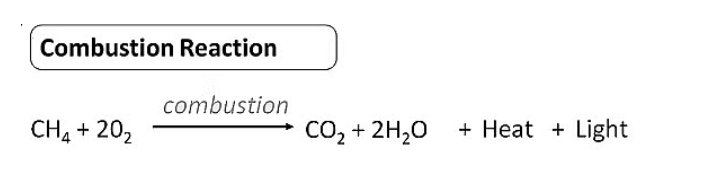
Combustion of Methane
CH4 + 2O2 → CO2(g) + 2H2O + energy
Substitution Reaction
Substitution Reaction
- These are the reactions in which one or more hydrogens of a hydrocarbon are replaced by some other atoms like chlorine .
- Substitution reactions are a characteristic property of Saturated hydrocarbons which are otherwise quite unreactive due to the presence of only C-C single bonds , but they are able to undergo substitution reactions.
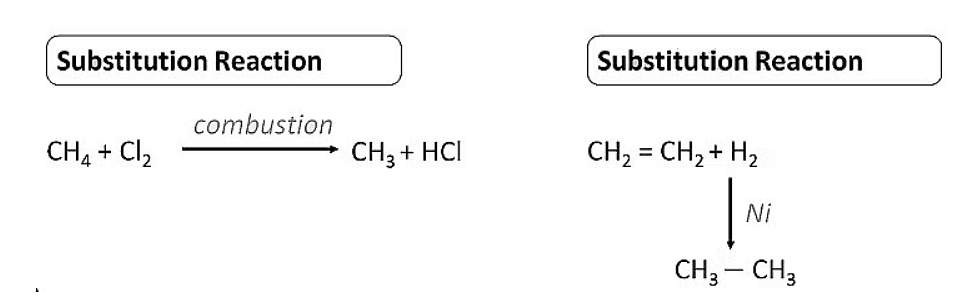
[Question: 906462]
Chlorination of methane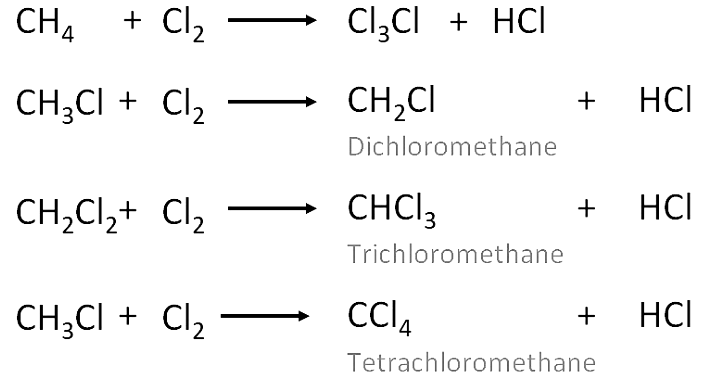
Addition Reaction
Addition Reaction
- These reactions involve the addition of one or more hydrogen atoms to double/triple bonds .
- Therefore, this is a characteristic reaction of unsaturated hydrocarbons . Saturated hydrocarbons don’t show addition reactions.
- For example, hydrogenation reactions .
Note: In this reaction Pd/C acts as a catalyst i.e. a substance to speed up the reaction.
Hydrogenation of Ethene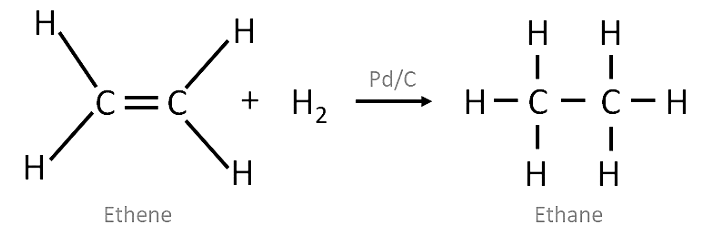
[Question: 906463]
|
80 videos|512 docs|74 tests
|
FAQs on Overview: Carbon Compounds & Covalent Bonding in Carbon Compounds - Science Class 10
| 1. What are carbon compounds and why are they important? |  |
| 2. What is a covalent bond and how does it relate to carbon compounds? |  |
| 3. What is the difference between non-polar and polar covalent compounds? |  |
| 4. What are organic compounds and how do they relate to carbon compounds? |  |
| 5. Why is carbon considered a versatile element in chemistry? |  |