Oxidation Number | Physical Chemistry for NEET PDF Download
| Table of contents |

|
| Oxidation Number |

|
| Important Rules for Determining Oxidation Number |

|
| Stock Notation |

|
| Solved Examples |

|
Oxidation Number
The oxidation number represents the charge a central metal atom maintains even after dissociating from all ligands attached to it. The oxidation state, or oxidation number, of an atom within a chemical compound explains the electron loss, indicating the degree of oxidation. It is a theoretical charge that an atom would carry if its bonds were entirely ionic.
- It shows the oxidation state of an element in a compound. It's determined based on a set of rules that consider the idea that in a covalent bond, the electron pair belongs completely to the more electronegative element.
- The oxidation number is defined as the charge that an atom appears to have when all other atoms are removed from it as ions. It may have a + or – sign.
- Oxidation number of an element may be a whole number (positive or negative) or fractional or zero.
Important Rules for Determining Oxidation Number
- In elements, when they are free or uncombined, each atom has an oxidation number of zero. For example, in H2, O2, Cl2, O3, P4, S8, Na, Mg, and Al, the oxidation number is zero.
- For ions with only one atom, the oxidation number equals the charge on the ion. So, Na+ has an oxidation number of +1, Mg2+ is +2, Fe3+ is +3, Cl– is –1, O2– is –2, and so on. In compounds, alkali metals always have an oxidation number of +1, alkaline earth metals have +2, and aluminum is considered +3 in all its compounds.
- Oxygen usually has an oxidation number of –2 in most compounds, but there are exceptions. In peroxides (e.g., H2O2, Na2O2), each oxygen atom has an oxidation number of –1, and in superoxides (e.g., KO2, RbO2), it's –(½). Rarely, in compounds with fluorine (e.g., OF2, O2F2), oxygen may have oxidation numbers of +2 and +1, respectively.
- Hydrogen typically has an oxidation number of +1, except when bonded to metals in binary compounds (containing two elements) like LiH, NaH, and CaH2, where its oxidation number is –1.
- Fluorine always has an oxidation number of –1 in its compounds. Other halogens (Cl, Br, I) also have –1 as oxidation numbers when they appear as halide ions. However, when combined with oxygen, for instance, in oxoacids and oxoanions, chlorine, bromine, and iodine may have positive oxidation numbers.
- The sum of oxidation numbers of all atoms in a compound must be zero. In a polyatomic ion, the sum of oxidation numbers of its atoms equals the charge on the ion. For example, in the carbonate ion (CO3)2–, the sum of oxidation numbers for three oxygen atoms and one carbon atom must equal –2.
Stock Notation
- The oxidation states of elements exhibiting variable oxidation states are specified by Roman numerals such as I, II, III, IV, etc., within parenthesis after the symbol or name of the element.
- This system was introduced for the first time by a German chemist, Alfred Stock, and is known as Stock notation. This may be illustrated as:
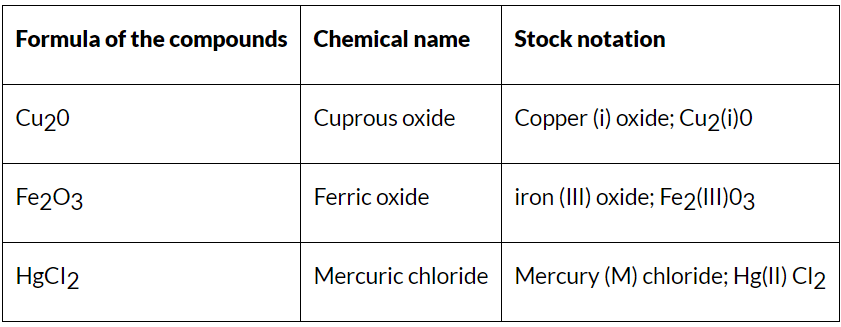 Some Examples of Stock Notation
Some Examples of Stock Notation
Solved Examples
Example: What is the oxidation state of sulphur in H2SO4 and H2SO5?
Solution: (i) H2SO4: Since there is no overall charge on the compound the oxidation states must cancel out. Although the oxidation state of hydrides is +1, there are two in the compound that must be accounted for. The same is true for oxygen; although the oxidation number of oxygen is -2, there are four oxygens present accounting for a total of -8. The +2 state contribution from the hydrides and -8 from the oxygens results in a -6 charge. The oxidation state on sulphur must be +6 for the molecule to be neutral.
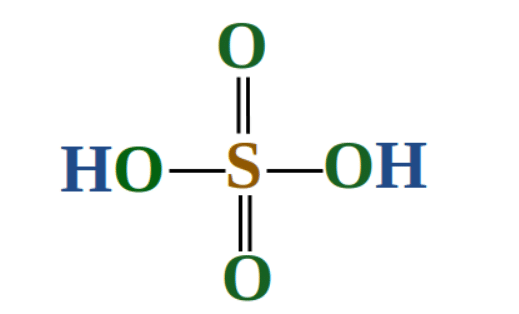 Structure of Sulphuric Acid
Structure of Sulphuric Acid
(ii) H2SO5: the oxidation number of S is +6 in H2SO5 due to the presence of a peroxy linkage bond in the molecule.
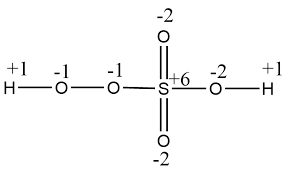 Structure of Caro's Acid
Structure of Caro's Acid
Example: What is the Oxidation Number of Nitrogen in Ammonium Nitrate i.e. NH4NO3?
Solution: The steps to find the Oxidation Number of Nitrogen in Ammonium Nitrate are:
- Split the molecule into two ions: Ammonium ion (NH4+) and Nitrate ion (NO3–).
- Determine the oxidation number of Nitrogen in each ion, denoted as X.
- For the Ammonium ion, where the overall charge is +1, set up the equation X + 4(+1) = +1.
- Solve the equation: X + 4 = 1 ⇒ X = -3.
- In the case of the nitrate ion, where the overall charge is -1 and oxygen has an oxidation number of -2, set up the equation X + 3(-2) = -1.
- Solve the equation: X – 6 = -1 ⇒ X = +5.
Therefore, the oxidation number of Nitrogen in ammonium nitrate is -3 and +5.
Example: Determine the oxidation number of each element in the following compounds:
(a) BaO2 (b) (NH4)2MoO4 (c)Na3Co(NO2)6 (d) CS2
Solution: (a) If oxygen in BaO2 had an oxidation number of -2, then barium would need to have an oxidation number of +4. However, elements in Group IIA, like barium, can't have a +4 oxidation state. This compound must be barium peroxide, [Ba2+][O22-]. Therefore, barium is +2 and oxygen is -1.
(b) In (NH4)2MoO4, the NH4+ ion has hydrogen at +1 and nitrogen at -3. With two NH4+ ions, the other part must be an MoO42- ion, where molybdenum is -6 and oxygen is -2.
(c) Sodium always has an oxidation state of +1 in its compounds. This compound contains the Co(NO2)63- complex ion, with six NO2- ions where nitrogen is +3 and oxygen is -2. The oxidation state of cobalt is thus +3.
(d) The most electronegative element in a compound always has a negative oxidation number. Since sulphur typically forms -2 ions, the oxidation number of sulphur in CS2 is -2, and carbon is +4.
Example: Calculate the oxidation number of nitrogen (N) in NO.
Solution: The oxidation state of oxygen(O) = -2
Consider the oxidation state of nitrogen(N) = x
Now, x-2 = 0 => x = +2
Hence, the oxidation number of N in NO is +2.
Example: Calculate the oxidation number of sulphur (S) in sulphuric acid:H2SO4
Solution: The oxidation state of oxygen(O) = -2
The oxidation state of hydrogen(H) = +1
Consider the oxidation state of sulphur (S) = X
Now,
2(+1)+X+(−22(+1)+X +(−2×4)
−2+X+8=0
X = +6
Hence, the oxidation number for sulphur in sulphuric acid is +6.
Example: Find oxidation number of Cr2 in the formula K2Cr2O7
Solution: K2= +2; O7= (-2 × 7); Cr2= 2 × x
Therefore, 2+ 2x-14=0. Thus x=+6 (Oxidation state of chromium)
Example: Find oxidation state of N in the formula NH4NO3
Solution: There are two different nitrogen atoms in the compound. Therefore we need to do the calculation separately
In NH+4, or X +4 (+1) = +1
Therefore x= -3 (Oxidation state in NH+4). The oxidation state of N in NO–3
y+3 × (-2)= -1
y= +6-1 =5
|
117 videos|223 docs|237 tests
|
FAQs on Oxidation Number - Physical Chemistry for NEET
| 1. What is an oxidation number? |  |
| 2. How is the oxidation number determined in a compound? |  |
| 3. Can an atom have multiple oxidation numbers? |  |
| 4. What is the significance of oxidation numbers in chemistry? |  |
| 5. How do oxidation numbers relate to redox reactions? |  |





















