Physical & Chemical Properties of Aldehydes, Ketones & Carboxylic Acids | Chemistry Class 12 - NEET PDF Download
(i) Physical Properties of Aldehydes and Ketones
State at Room Temperature:
- Methanal is a gas.
- Ethanal is a liquid that evaporates easily.
- Other aldehydes and ketones are usually liquid or solid.
Boiling Points:
- Aldehydes and ketones have higher boiling points than similar-sized hydrocarbons and ethers due to weak attractions between molecules (dipole-dipole interactions).
- However, their boiling points are lower than alcohols of similar size because they lack hydrogen bonding between molecules.
Solubility:
- Lower aldehydes and ketones like methanal, ethanal, and propanone mix well with water because they can form hydrogen bonds with water molecules.
- Solubility decreases as the alkyl chain lengthens.
- Aldehydes and ketones dissolve well in organic solvents like benzene, ether, methanol, and chloroform.
Odour:
- Lower aldehydes have strong, sharp smells.
- As molecules get bigger, the smell becomes less strong and more pleasant.
- Many natural aldehydes and ketones are used in making perfumes and flavors.
(ii) Chemical Reactions of Aldehydes and Ketones
1. Nucleophilic Addition Reactions
(i) Mechanism:
- Instead of electrons attacking, a nucleophile (like a negatively charged ion) attaches to the carbon atom in the carbonyl group.
- This changes the carbon's structure from flat to more three-dimensional.
- A temporary intermediate, called an alkoxide, is formed.
- A proton (H+) from the environment combines with this intermediate to create a neutral product.
- The end result is the addition of the nucleophile and a proton across the carbon-oxygen double bond.
(ii) Reactivity:
- Aldehydes are usually more reactive than ketones in these reactions.
- This is because ketones have bulkier groups around the carbonyl carbon, making it harder for the nucleophile to approach.
- Also, aldehydes are more eager to react because they have fewer alkyl groups, which reduces the carbon's positive charge more effectively.
(iii) Examples of Reactions:
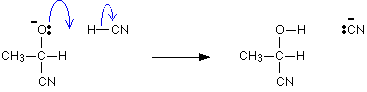
(a) Addition of Hydrogen Cyanide (HCN):
- Aldehydes and ketones react with hydrogen cyanide (HCN) to form cyanohydrins.
- This reaction is slow with pure HCN, so it's usually catalyzed by a base.
- Cyanide ions (CN-) formed act as strong nucleophiles, readily adding to carbonyl compounds to create cyanohydrins.
- Cyanohydrins are useful intermediate compounds in synthesis.
(b) Addition of Sodium Hydrogensulphite:
- Sodium hydrogensulphite adds to aldehydes and ketones, forming addition products.
- The equilibrium usually favors the right side for aldehydes and the left side for ketones due to steric reasons.
- These addition compounds dissolve in water and can be converted back to the original carbonyl compound by treating them with weak acids or alkalis.
- This property makes them useful for separating and purifying aldehydes.
(c) Addition of Grignard Reagents:

(d) Addition of Alcohols:
- Aldehydes react with monohydric alcohols in the presence of dry hydrogen chloride.
- This forms an intermediate called a hemiacetal, which further reacts with more alcohol to produce a gem-dialkoxy compound known as an acetal.
- Ketones react similarly with ethylene glycol, forming cyclic products called ethylene glycol ketals.
- Dry hydrogen chloride makes the carbonyl carbon more electrophilic, facilitating the nucleophilic attack of the alcohol.
- Acetals and ketals can be converted back to aldehydes and ketones respectively by hydrolysis with aqueous mineral acids.
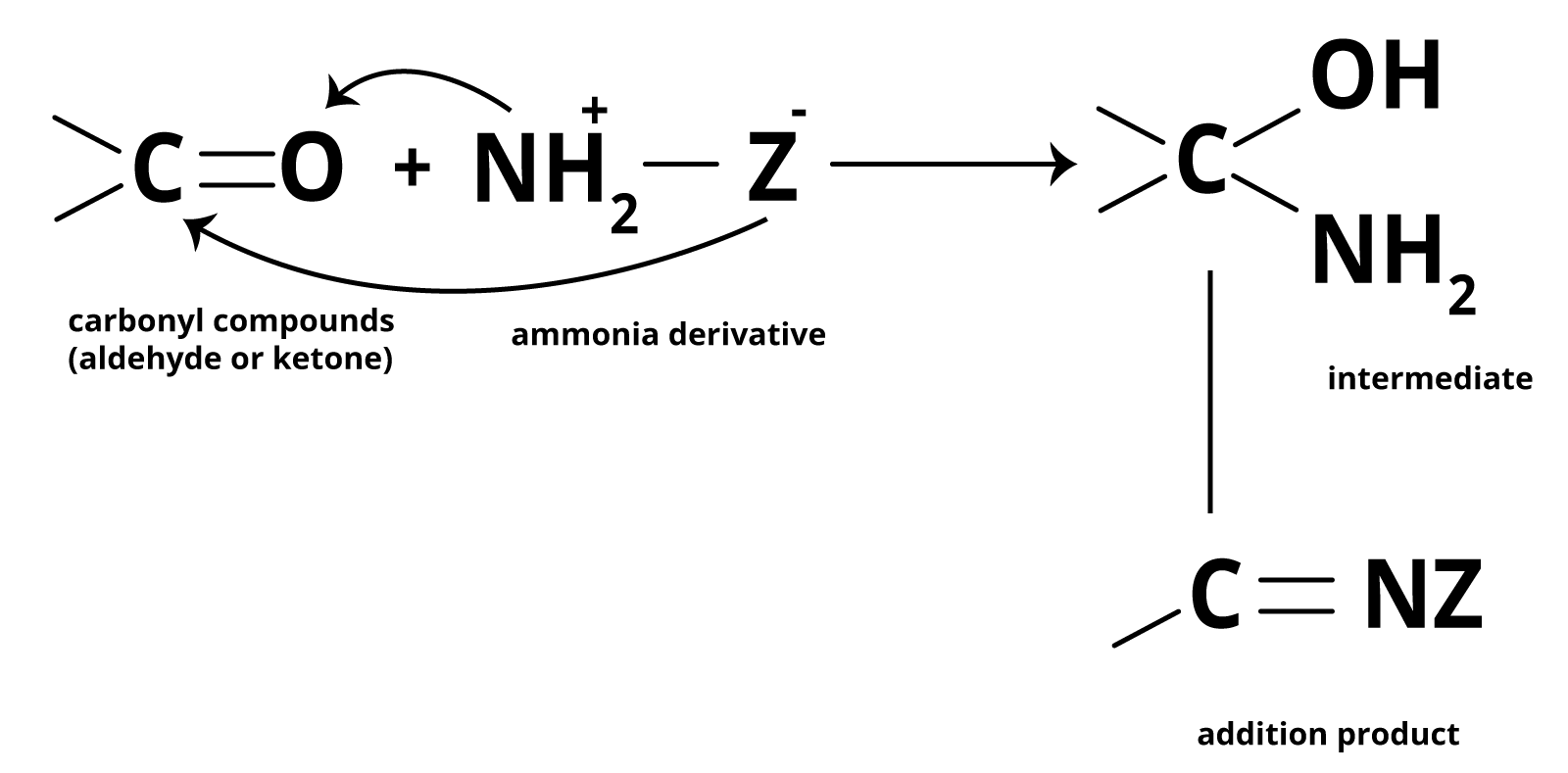
(e) Addition of Ammonia and its Derivatives:
- Nucleophiles like ammonia and its derivatives (H2N-Z) add to the carbonyl group of aldehydes and ketones.
- This reversible reaction, catalyzed by acid, favors product formation due to rapid dehydration of the intermediate to form imines (>C=N-Z).
2.Reduction
(i) Reduction to Alcohols:
- Aldehydes turn into primary alcohols, while ketones become secondary alcohols.
- This change happens when we use substances like sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4), or by catalytic hydrogenation (refer to Unit 7, Class XII).
(ii) Reduction to Hydrocarbons:
- Aldehydes and ketones can also be reduced to hydrocarbons.
- This means their carbonyl groups are changed into CH2 groups.
- We can do this by treating them with zinc amalgam and concentrated hydrochloric acid (known as Clemmensen reduction), or with hydrazine followed by heating with sodium or potassium hydroxide in a high-boiling solvent like ethylene glycol (known as Wolff-Kishner reduction).
3.Oxidation
Aldehydes vs. Ketones:
- Aldehydes are easily oxidized to carboxylic acids using common oxidizing agents like nitric acid, potassium permanganate, and potassium dichromate.
- Even milder agents like Tollens’ reagent and Fehlings’ reagent can oxidize aldehydes.
- Ketones, on the other hand, require stronger oxidizing agents and higher temperatures for oxidation. Their oxidation breaks carbon-carbon bonds, producing a mix of carboxylic acids with fewer carbon atoms.
Tests to Distinguish Aldehydes and Ketones:
1.Tollens’ Test:
- When warmed with ammoniacal silver nitrate solution (Tollens’ reagent), aldehydes produce a shiny silver mirror. This indicates oxidation to carboxylate anions in an alkaline environment.
2. Fehling’s Test:
- Mixing Fehling solution A (copper sulfate) with Fehling solution B (alkaline sodium potassium tartrate) forms Fehling’s reagent.
- Heating an aldehyde with Fehling’s reagent produces a reddish-brown precipitate, indicating oxidation to carboxylate anions. Aromatic aldehydes don't react with this test.
3. Oxidation of Methyl Ketones by Haloform Reaction:
- Aldehydes and ketones with a methyl group linked to the carbonyl carbon (methyl ketones) are oxidized by sodium hypohalite.
- This converts the methyl group into a haloform and reduces the carbonyl compound to a carboxylic acid with one fewer carbon atom.
- This oxidation doesn't affect any carbon-carbon double bond present in the molecule.
4. Iodoform Reaction:
The iodoform reaction with sodium hypoiodite is also used to detect the presence of CH3CO or CH3CH(OH) groups, which produce CH3CO groups upon oxidation.
5. Reactions Due to α-Hydrogens
Acidity of α-Hydrogens:
- The α-hydrogens in aldehydes and ketones are slightly acidic.
- This is because the carbonyl group pulls electron density away from them, making them more prone to lose a proton.
- Also, the resulting negative charge is stabilized by resonance.
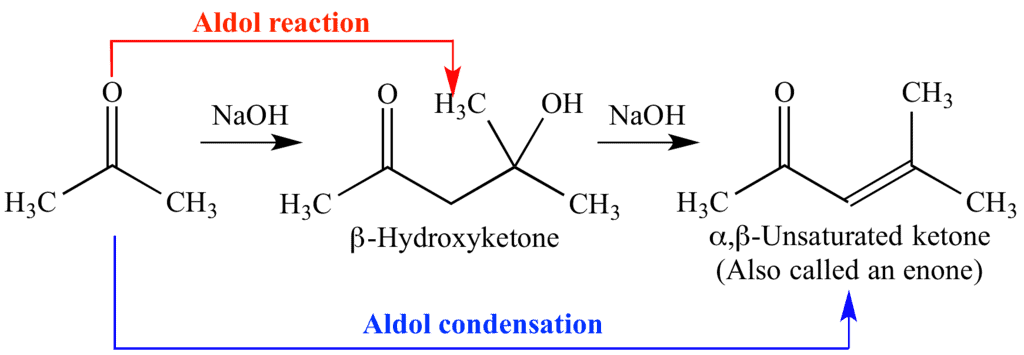
(i). Aldol Condensation:
- Aldehydes and ketones with α-hydrogens react in the presence of a weak alkali catalyst.
- This reaction forms β-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), depending on the starting compound.
- It's called Aldol because it combines the words "aldehyde" and "alcohol," reflecting the groups present in the products.
- The aldol or ketol can lose water to form α,β-unsaturated carbonyl compounds, which are aldol condensation products.
(ii). Cross Aldol Condensation:
- When different aldehydes or ketones with α-hydrogens react together in aldol condensation, it's called cross aldol condensation.
- If both reactants have α-hydrogens, they produce a mixture of four products.
- For example, mixing ethanal and propanal in aldol condensation would yield four different products.
6. Other Reactions
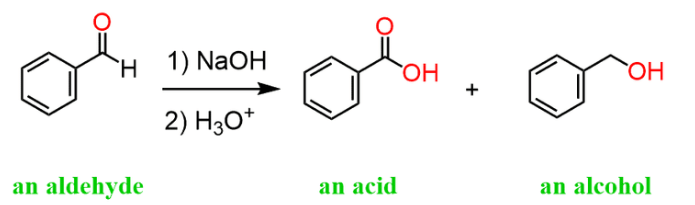
i) Cannizzaro Reaction:
- Aldehydes without α-hydrogen atoms undergo a special reaction called the Cannizzaro reaction.
- When heated with concentrated alkali, these aldehydes undergo self-oxidation and reduction (disproportionation).
- In this process, one aldehyde molecule gets reduced to an alcohol while another gets oxidized to a carboxylic acid salt.
(ii) Electrophilic Substitution Reaction:
- Aromatic aldehydes and ketones can undergo a type of reaction called electrophilic substitution.
- In this reaction, the carbonyl group in the aromatic ring acts as a deactivating and meta-directing group.
- This means it influences the way new atoms or groups are added to the aromatic ring, typically directing them to the meta position.
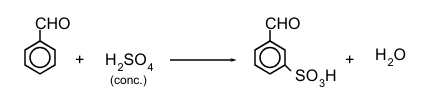
Carboxylic Acids
(i) Physical properties of acids and acid derivatives
1. Boiling point :
The high boiling points of carboxylic acids is the result of formation of a stable hydrogen-bonded dimer.
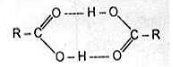
Hydrogen bonded acid dimer
2. Solubility :
Carboxylic acids form hydrogen bonds with water and the lower molecular –weight carboxylic acids (upto 4 carbon atoms) are miscible with water.
Acid derivatives (esters, acid chlorides, anhydride, nitriles and amides) are soluble in organic solvents such as alcohols, ethers, chlorinated alkanes and aromatic hydrocarbons.
Methods of preparation of carboxylic acids
1. Synthesis of carboxylic acids by the carboxylation of Grignard's reagent:
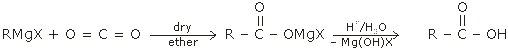
2. Synthesis of Carboxylic acids by the hydrolysis of nitriles:

Nitrile carboxylic acid
(ii) Chemical Reactions
1. Acidic strength %
Acidity of carboxylic acids :-
Ex.
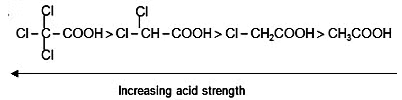
Ex. HCOOH > CH3COOH > CH3-CH2-COOH
Ex. 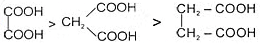
Ex. Relative acid strength is:-
RCOOH > HOH > ROH > HC = CH > NH3 > RH
Note:- Acidity of acids is compared by comparing stability of conjugate base.
2. Reaction involving removal of proton from –OH group.
1.Action with blue litmus : All carboxylic acids turn blue litmus red.
2.Reaction with metals :
2 CH3 COOH + 2Na → 2CH3COONa + H2
Sodium acetate
2CH3 COOH + Zn → (CH3COO)2 Zn + H2
Zinc acetate
3.Reaction with alkalies:
CH3 COOH + NaOH → CH3 COONa + H2O4.Reaction with carbonates and bicarbonates :
2CH3COOH + Na2CO3 → 2CH3 COONa + CO2 + H2O
CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O
Reaction of carboxylic acid with aqueous sodium carbonate solution produces brisk effervescence. However, most phenols do not produce effervescence. Therefore, the reaction may be used to distinguish between carboxylic acids and phenols.
5.Reaction with Grignard's reagent :
Note: A stronger acid displaces a weaker acid from salt of the weaker acid.
Ex. CH3COOH (Stronger acid) + CH3ONa → CH3 COONa + CH3 —OH (WeakerAcid)
Ex. CH3COOH (stronger acid) + NaHCO3 → CH3 COONa + H2 CO3 (Weaker acid) → H2O + CO2
3. Reaction involving replacement of –OH group
(i) Formation of acid chlorides :
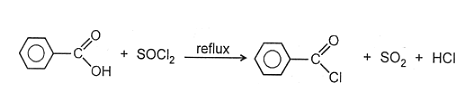
(2) Fisher Esterification:
Carboxylic acid react with alcohol to form esters through a condensation reaction known as esterification.
General Reaction :
Specific Example:
Mechanism:
Acid catalysed esterfication:
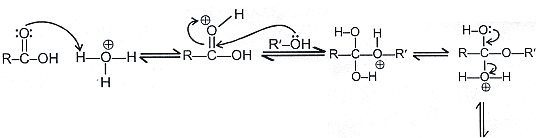
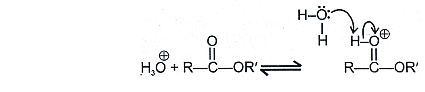
If we follow the forward path in this mechanism, we have the mechanism for the acid catalysed esterification for an acid. If however, we follow the reverse reactions, we have the mechanism for the acid catalysed hydrolysis of an ester. Acid catalysed ester hydrolysis gives:
(3) Formation of amides:
(4) Formation of acid anhydride:
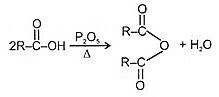
Decarboxylation reactions:
1. Soda-lime decarboxylation :
General reaction:
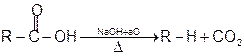
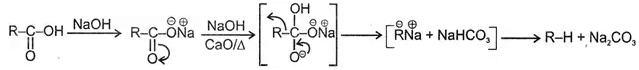
2. Decarboxylation of β- keto carboxylic acids :
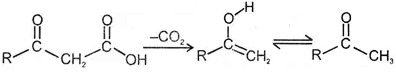
 - keto acid
- keto acid
3. Kolbe's electrolysis:
(II) R. + R. → R - R
If n is the number of carbon atoms in the salt of carboxylic acid, the alkane formed has 2(n-1) carbon atoms.
4. Hunsdiecker Reaction (Brome-decarboxylation):
R-COOAg + Br2 → R-Br + CO2 + AgBr
Mechanism:
5. HVZ Reaction (Halogenation of aliphatic acids and Substituted acids):
Carboxylic Acid Derivatives
Closely related to the carboxylic acids and to each other are a number of chemical families known as functional derivatives of carboxylic acids : acid chloride, anhydrides, amides, and esters. These derivatives are compounds in which the -OH of a carboxyl group has been replaced by–Cl, -OOCR, -NR2 or –OR.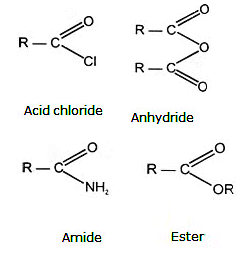
They all contain the acyl group ,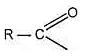
(A) Acid halides:
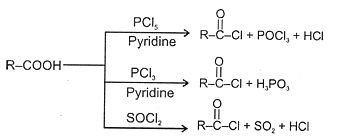
Methods of preparation of Acyl halides:
(A) Acid halides:
(i) RCOOH + PCl5 → RCOCl + POCl3 + HCl
(ii) 3RCOOH + PCl3 → 3RCOCl + H3PO3
(iii) RCOOH + SOCl2 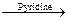 RCOCl +SO2 + HCl
RCOCl +SO2 + HCl
Ex.
Ex.
Chemical Reactions:
1. Reaction with carboxylic acids:
2. Reaction with alcohols:
Acyl chlorides react with alcohols to form esters. The reaction is typically carried out in the presence of pyridine.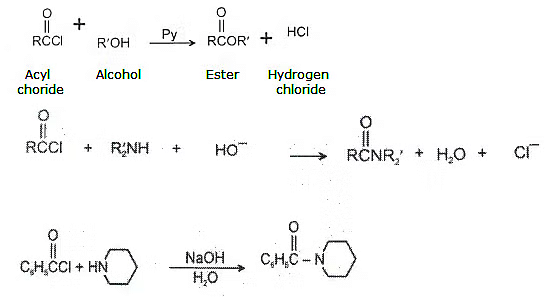
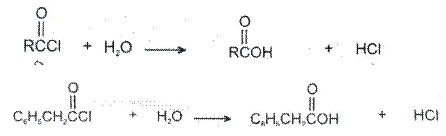
3. Hydrolysis:
4. Reaction of acid halide with organometallic compounds:
(a) With Grignard's reagent-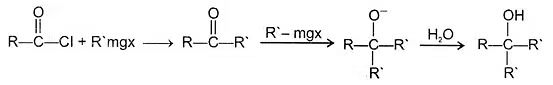
(b) Reaction with Gilmann's reagent-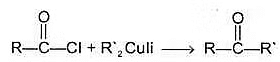
5. Reduction of acid halide:
(a) Reduction LiAlH4 -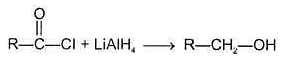
(b) Reduction with H2/Pd/BaSO4 Rosenmund reduction-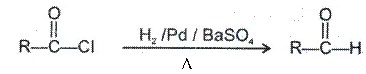
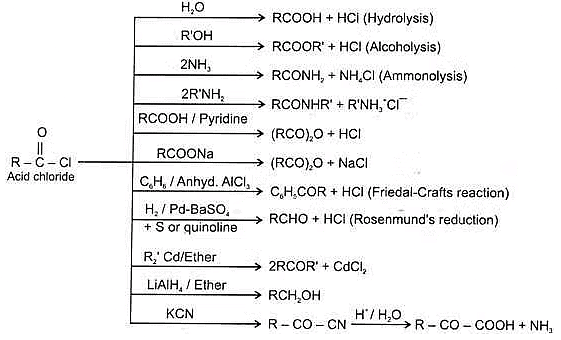
(B) Acid amides
Methods of preparation of acids amides:
(B) Acid amides
1. By reaction of esters with ammonia and amines.

Ammonia is more nucleophilic than water, making it possible to carry out this reaction using aqueous ammonia.
Ex.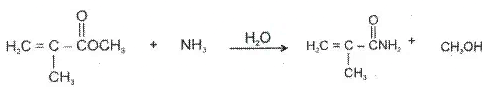
2. From acid halides:
RCOCl + 2NH3 → RCONH2 + NH4Cl
3. From anhydride:
(RCO)2O + 2NH3 → RCONH2 + RCOO NH4
4. From esters:
RCOOR + NH3 → RCONH2 + R’OH
5. From ammonium salt of carboxylic acid:
RCOONH4  RCONH2 + H2O
RCONH2 + H2O
CH3 COONH4  CH3CONH2
CH3CONH2
6. From cyanides:
7.
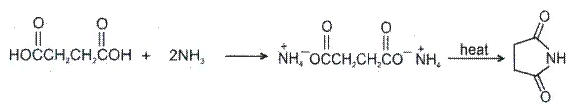
Chemical Reactions:
(1) Hoffmann rearrangement:
General reaction-
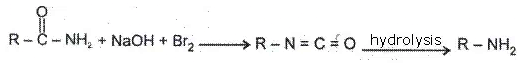
(2) Hydrolysis of amides:
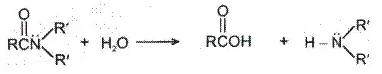
In acid, however, the amine is protonated, giving an ammonium ion, R2’ 

Summary of Reaction of Amide:
(C) Esters
Methods of Preparation:
(i) CH3 COOH + C2H5OH 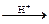 CH3COOC2 H5 + H2O
CH3COOC2 H5 + H2O
Acetic acid
C6H5COOH + CH3OH 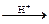 C6H5 COOCH3 + H2O
C6H5 COOCH3 + H2O
(ii) CH3 COCl + C2H5OH 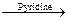 CH3COOC2H5 + HCl
CH3COOC2H5 + HCl
Alcohols react with acyl chlorides by nucleophilic acyl substitution to yield esters. These reactions are typically performed in the presence of a weak base such as pyridine.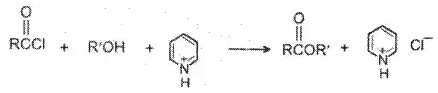
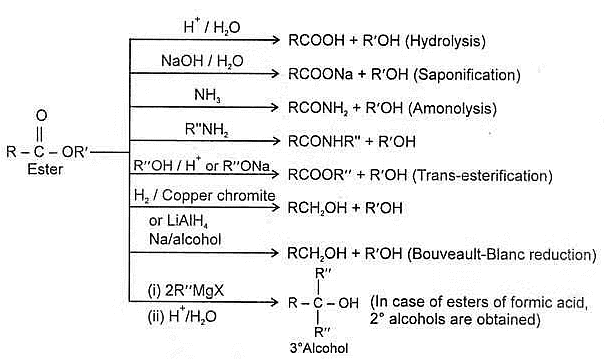
Summary of reaction of esters :
(D) Acid anhydrides
Methods of Preparation of acid anhydrides:
1. From carboxylic acids
Ex.
CH3COOH + HOOCCH3 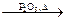 CH3CO.O.COCH3 + H2O
CH3CO.O.COCH3 + H2O
Acetic acid Acetic anhydride
Ex.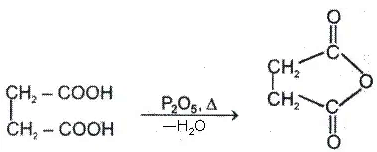
Ex.
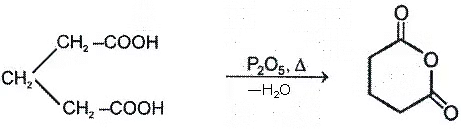
2. From acid and acid halide
Ex. CH3COOH + CH3COCl 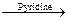 CH3 CO.O.COCH3 + HCl
CH3 CO.O.COCH3 + HCl
Ex. CH3COCl + CH3COONa 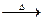 CH3CO.O.COCH3 + NaCl
CH3CO.O.COCH3 + NaCl
Chemical Reactions:
1. Reaction with aromatic compounds (Friedel crafts acylation)-

2. Reaction with alcohols-
Ex:
3. Reaction with ammonia and amines-
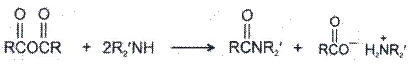
4. Hydrolysis-
Acid anhydrides react with water to yield two carboxylic acids. Cyclic anhydrides yield dicarboxylic acids.
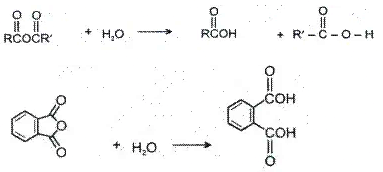
5. Heating Effects-
a. Heating effect on monocarboxylic acid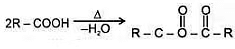
b. Heating effect on dicarboxylic acid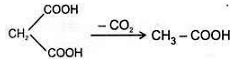
c. Heating effect on Hydroxy acids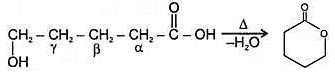
1.  Hydroxy acid
Hydroxy acid
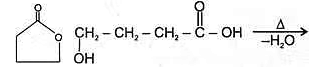
2. 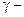 Hyroxy acid
Hyroxy acid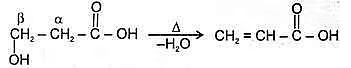
3. 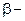 Hydroxy acid
Hydroxy acid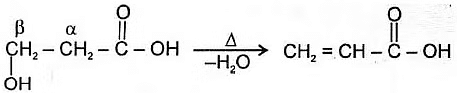
Since 4 or 8 membered rings are less stable therefore β-Hydroxy acids on heating produce α,β unsaturated carboxylic acid.
4. An α -Hyroxy acid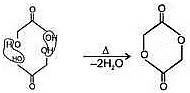
5. Heating effect on esters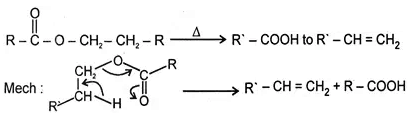
This reaction follows syn elimination & Hoffman product is formed.
|
75 videos|278 docs|78 tests
|
FAQs on Physical & Chemical Properties of Aldehydes, Ketones & Carboxylic Acids - Chemistry Class 12 - NEET
| 1. What are the physical properties of aldehydes and ketones? |  |
| 2. What are some common chemical reactions of aldehydes and ketones? |  |
| 3. How are carboxylic acids prepared? |  |
| 4. What is Fisher Esterification? |  |
| 5. What are some common decarboxylation reactions involving carboxylic acids? |  |