Revision Notes: Electrochemistry | Chemistry Class 12 - NEET PDF Download
Introduction to Electrochemistry
Electrochemistry deals with the relationship between electrical energy and chemical changes. It studies the production of electricity from spontaneous chemical reactions and the use of electrical energy to drive non-spontaneous reactions. Electrochemical cells, batteries, fuel cells, and electrolysis are practical applications of electrochemistry.
Types of Electrochemical Cells
- Galvanic Cells (Voltaic Cells): These cells convert chemical energy of spontaneous redox reactions into electrical energy. Example: Daniell cell.
- Electrolytic Cells: These use electrical energy to carry out non-spontaneous chemical reactions. Example: Electrolysis of water.
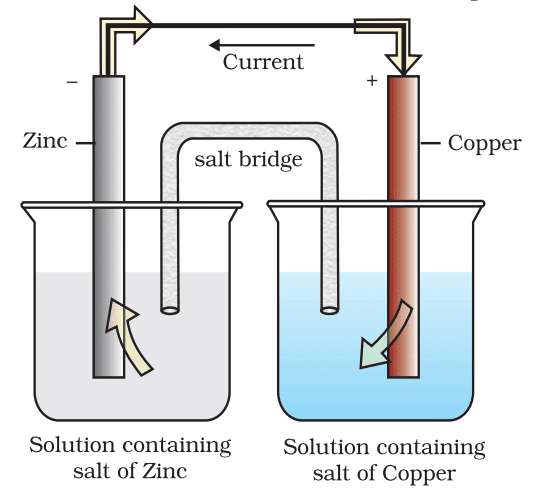
Galvanic (Voltaic) Cells
A galvanic cell consists of two half-cells connected by a salt bridge or porous partition. Each half-cell contains an electrode immersed in an electrolyte. The electrons flow from the anode (oxidation) to the cathode (reduction), generating electric current.
Electrode Potential and Standard Electrode Potential
- Electrode potential is the potential difference between the electrode and the solution in which it is immersed.
- Standard electrode potential (E°) is measured under standard conditions (298 K, 1 M concentration, 1 atm pressure).
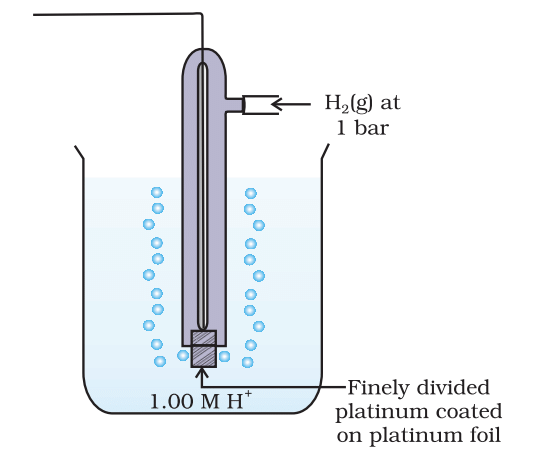
The standard electrode potential of a half-cell is determined relative to the standard hydrogen electrode (SHE), which is assigned a potential of 0 V.
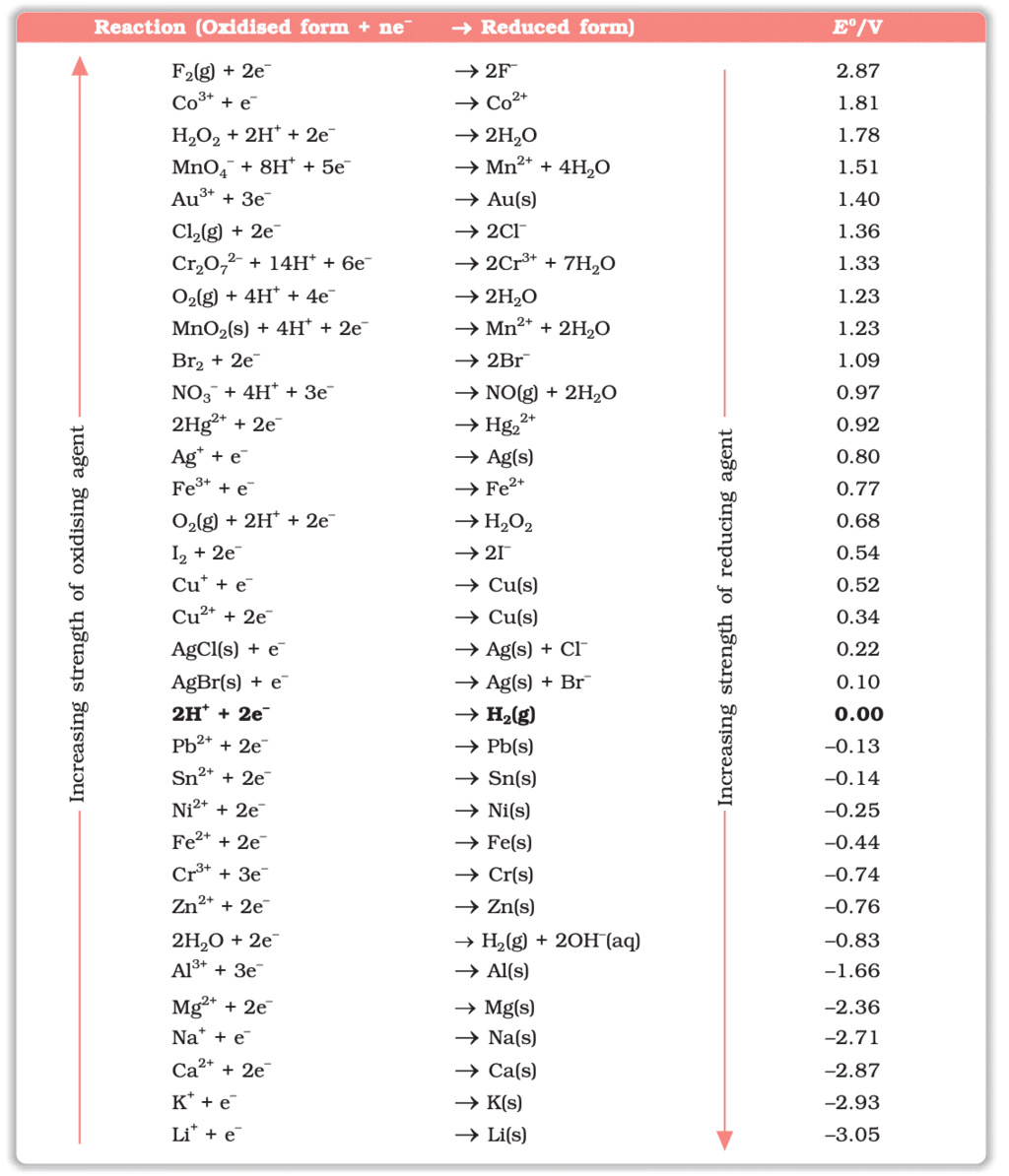
The standard electrode potentials for various half-reactions are listed in a table. These values help in predicting the direction of the redox reactions and the feasibility of electrochemical processes. For example:
Table: Standard Electrode Potentials at 298
Gibbs Free Energy and Cell Potential
The Gibbs free energy change (ΔG) of a redox reaction is related to the cell potential (E) by the equation:
ΔG = -nFE
Where n is the number of moles of electrons transferred, F is Faraday's constant, and E is the cell potential. A positive E indicates a spontaneous reaction.
The Nernst Equation
The Nernst equation is used to calculate the cell potential under non-standard conditions, taking into account the concentrations of the ionic species involved in the reactions. It is given by:
E(cell) = E°(cell) - (RT/nF) * ln(Q)
Where:
- E°(cell) = standard cell potential
- R = universal gas constant
- T = temperature in Kelvin
- n = number of moles of electrons exchanged
- F = Faraday's constant
- Q = reaction quotient (products over reactants)
Faraday’s Laws of Electrolysis
- First Law: The amount of substance deposited or dissolved at an electrode is directly proportional to the quantity of electricity passed through the electrolyte.
- Second Law: The amounts of different substances deposited or dissolved by the same quantity of electricity are proportional to their chemical equivalent weights.
Electrolytic Cells
In electrolytic cells, electrical energy is used to drive non-spontaneous reactions. Examples include the electrolysis of water, NaCl, and the extraction of metals like aluminum from bauxite.
Applications of Electrochemistry
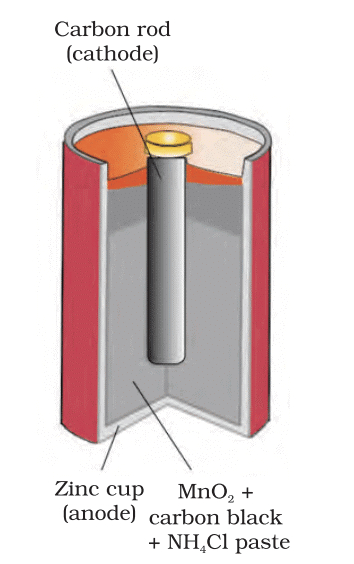
- Batteries: Convert chemical energy into electrical energy. Examples include dry cells, lead-acid batteries, and lithium-ion batteries.
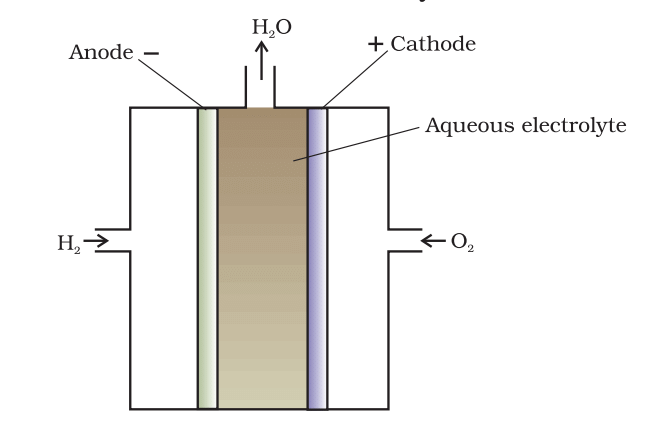
- Fuel Cells: Convert chemical energy into electrical energy through the reaction of hydrogen and oxygen, producing water as the only byproduct.
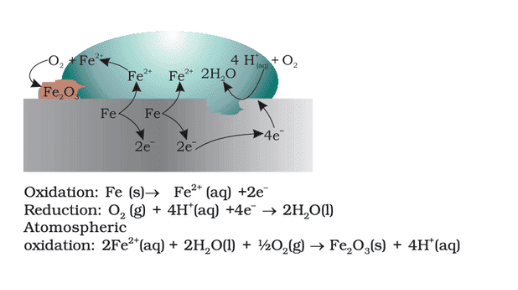
- Corrosion: The electrochemical oxidation of metals, especially iron, leads to rust formation.
|
75 videos|278 docs|78 tests
|
FAQs on Revision Notes: Electrochemistry - Chemistry Class 12 - NEET
| 1. What is electrochemistry and why is it important for NEET? |  |
| 2. What are the types of electrochemical cells? |  |
| 3. How do you calculate the cell potential in electrochemical cells? |  |
| 4. What is the significance of standard reduction potentials in electrochemistry? |  |
| 5. Can you explain the concept of electrolysis and its applications? |  |
















