Revision Notes: Principles Related to Practical Chemistry | NCERT Exemplar & Revision Notes for NEET PDF Download
QUALITATIVE ANALYSIS OF ORGANIC COMPOUNDS
This is used to detect nitrogen, halogen and sulphur present in organic compound.
(a) Sodium Extract: Aqueous solution containing soluble sodium salt of the elements i.e NaCl, Na2S and NaCNS formed by fusion of compound with sodium metal.
(b) Formation of Sodium Extract: It is a two step process
Step 1: Organic compounds are fused with dry sodium in a fusion-tube
Step 2: Fused mass after extraction with water is boiled and filtered.
(c) Use of Sodium Extract: Sodium extract (S.E.) is used to detect elements (other than C and H) and the tests are given in the table.
| Element | Sodium Extract (S.E.) | Confirmed Test |
| Nitrogen | Na + C + N +Δ→ NaCl | S.E.+ FeSO4 +NaOH, boil and cool + FeCl3 + conc. HCl → Blue/ green colour Reactions Involved: 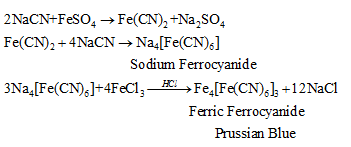 |
| Sulphur | 2Na + S → Na2S | (i) S.E. + sodium nitroprusside → Violet Colour (ii) S.E + CH3CO2H + (CH3CO2)2 Pb → black ppt. Reactions Involved: 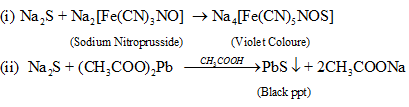 |
| Halogen | Na +X + Δ→ NaX (X = Cl, Br, I) | S.E. + HNO3+AgNO3→ (i) White ppt soluble in aq NH3 confirms Cl. (ii) Pale yellow ppt partially soluble in aq. NH3 confirms Br. (iii) Yellow ppt insoluble in aq. NH3 confirms I. Reactions Involved:  |
| Nitrogen and sulphur together | Na+ C + N + S+ Δ→ NaCNS with excess of Na the thiocyanate formed decomposes into cyanide and sulphide. NaCNS + 2Na → NaCN +Na2S | As in test for nitrogen; instead of green or blue colour, blood red colouration confirms presence of N and S both. Reactions Involved: 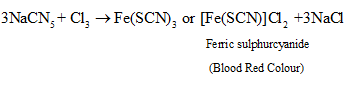 |
QUALITATIVE ANALYSIS OF INORGANIC SALTS
1. Physical Examination of Salts/Mixture
| Observation | Inference |
| 1. Substance is coloured | |
| (i) Blue | Copper salt |
| (ii) Dark green | Chromium salt |
| (iii) Green | Salts of Fe(II), Ni, Cu or Cr |
| (iv) Light yellow or brown | Salts of Fe(III) |
| (v) Dark brown | PbO2,Bi2S3 |
| (vi) Light pink | Salts of Mn |
| (vii) Pink | Salts of Co |
| (viii) Red | HgO, HgI2,Pb3O4 |
| (ix) Orange red | Sb2S3 |
| 2. Substance is deliquescent | CaCl2,ZnCl2,MgCl2, MnCl2, nitrites, nitrates |
| 3. Substance is heavy | Salts of Pb, Hg and Ba |
| 4. Substance is light | Carbonates of Bi, Mg, Al, Zn, Ca, Sr |
2. Effect of Heating
| Observation | Inference |
| 1. Substance melts | Salts of alkali metals and salts having water of crystallisation. |
| 2. Substance decripitates (crackling noise) | NaCl, KI, Pb(NO3)2 and Ba(NO3)2 |
| 3. Substance swells (due to loss of water of crystallisation) | Alums, borates and phosphates |
| 4. The substance sublimes and the colour of sublimate is | |
| (i) White | HgCl2, Hg2Cl2,NH4X, AlCl3, As2O3, Sb2O3 |
| (ii) Yellow | As2S3 and HgI2 (turns red when rubbed with glass rod). |
| (iii) Blue black and violet vapours | Iodides |
| 5. A residue (generally oxide) is left and its colour is | |
| (i) Yellow (hot) and white (cold) | ZnO |
| (ii) Reddish brown (hot); yellow (cold) | PbO |
| (iii) Black (hot); Red (cold) | HgO, Pb3O4 |
| (iv) Black (hot); Red brown (cold) | Fe2O3 |
| 6. Gas is evolved | |
| (A) Colourless and odourless | |
| (i) O2 - rekindles a glowing splinter | Alkali nitrates (2KNO3 → 2KNO2 + O2) |
| (ii) CO2 - turns lime water milky | Carbonates and oxalates (CaCO3 → CaO + CO2) |
| (iii) N2 | Ammonium nitrite (NH4NO2 → N2 + 2H2O) |
| (B) Colourless gas with odour | |
| (i) NH3 - Turns red litmus blue and mercurous nitrate paper black | Ammonium salts (NH4)2SO4 → NH4HSO4 + NH3 |
| (ii) SO2 - Smell of burning sulphur, turns acidified K2Cr2O7 paper green | Sulphites and thiosulphates CaSO3 →CaO + SO2 |
| (iii) HCl - Pungent smell, white fumes with ammonia | Hydrated chlorides CaCl2.6H2O → Ca(OH)2 + 4H2O + 2HCl |
| (iv) H2S - smell of rotten eggs, turns lead acetate paper black | Sulphides Na2S + 2H2O → 2NaOH + H2S |
| (C) Coloured gas | |
| (i) NO2 - Brown, turns starch iodide paper blue | Nitrites and nitrates of heavy metals 2Cu(NO3)2 → 2CuO + 4NO2 + O2 |
| (ii) Br2 - Reddish brown | Bromides 2CdBr2 + O2 → 2CdO + 2Br2 |
| (A) Turns starch paper yellow | |
| (B) turns starch iodide paper blue | |
| (iii) I2 - Violet, turns starch paper blue | Iodides 2CdI2 + O2 → 2CdO + 2I2 |
| (iv) Cl2 - Greenish yellow | Chlorides |
| (A) bleaches moist litmus paper | CuCl2 + H2O → CuO + 2HCl CuO + 2HCl → Cu + H2O + Cl2 |
| (B) bleaches indigo solution | |
| (C) turns starch iodide paper blue |
3. Flame test
| Metals | Colour |
| Li | crimson red |
| Na | golden yellow |
| K | Violet |
| Ca | Brick red |
| Sr | Crimson |
| Ba | apple green |
TEST FOR ANIONS
1. Carbonate (CO32- )
(i) Dilute HCl : gives effervescence, due to the evolution of carbon dioxide.
CO32- + 2H+ → CO2 + H2O
The gas gives turbidity with lime water and baryta water.
CO2 + Ca2+ + 2OH- → CaCO3 ¯ + H2O
CO2 + Ba2+ + 2OH-→ BaCO3 ¯ + H2O
On prolonged passage of carbon dioxide in lime water, the turbidity slowly disappears due to the formation of soluble hydrogen carbonate.
CaCO3 ¯→ + CO2 + H2O → Ca(HCO3)2
(ii) Barium chloride or Calcium chloride solution: White ppt of barium or calcium carbonate is obtained, which is soluble in mineral acid.
CO32- + Ba2+ → BaCO3 ¯
CO32- + Ca2+ → CaCO3 ¯
(iii) Silver nitrate solution: White ppt of silver carbonate is obtained.
CO32- + 2Ag+ → Ag2CO3¯
The ppt so obtained is soluble in nitric acid and in ammonia. The ppt becomes yellow or brown on addition of excess reagent and same may also happen if the mix is boiled, due to the formation of silver oxide
Ag2CO3¯ → Ag2O ¯ + CO2
2. Sulphites (SO32-)
(i) Dilute HCl or Dilute H2SO4: decomposes with the evolution of sulphur dioxide
SO32- + 2H+ → SO2 + H2O
The gas has a suffocating odour of burning sulphur.
(ii) Acidified potassium dichromate solution: Turns filter paper moistened with acidified potassium dichromate solution, green due to the formation of Cr3+ions.
(iii) Lime water: On passing the gas through lime water, a milky ppt is formed.
Precipitate dissolves on prolonged passage of the gas, due to the formation of hydrogen sulphite ions.
(iv) Barium chloride or Strontium chloride solution: Gives white ppt. of barium or strontium sulphite.
3. Sulphide (S-2)
(i) Dil. HCl or Dil. H2SO4: A colourless gas smelling of rotten eggs (H2S) is evolved.
S2- + 2H+ → H2S
(ii) The gas turns lead acetate paper black
(iii) Gives yellow ppt. with CdCO3
Na2S + CdCO3 → CdS¯ + Na2CO3
(iv) Silver nitrate solution: black ppt. of silver sulphide insoluble in cold but soluble in hot dil nitric acid.
S2- + 2Ag+ → Ag2S ¯
(v) Sodium nitroprusside solution: Turns sodium nitroprusside solution purple
Na2S + Na2[Fe(CN)5NO] → Na4[Fe(CN)5NOS]
4. Nitrites (NO2-)
(i) Dil HCl and Dil. H2SO4: Adding to solid nitrite in cold yields pale blue liquid (due to the presence of free nitrous acid HNO2 or its anhydride N2O3) & the evolution of brown fumes of nitrogen dioxide, the latter being largely produced by combination of nitric oxide with the oxygen of the air
(ii) Silver nitrate solution: White crystalline ppt. is obtained
NO2- + Ag+ → AgNO2¯
(iii) Turns acidified KI - starch paper blue
2KI + 2NO2 → 2KNO2 + I2
Starch + I2 → Blue colour
(iv) Brown ring test: When the nitrite solution is added carefully to a conc. solution of Iron(II) sulphate acidified with dil acetic acid or with dilute sulphuric acid, a brown ring is formed, due to the formation of [FeNO]SO4 at the junction of the two liquids.
5. Acetate (CH3COO-)
(i) Dilute Sulphuric Acid: Smell of vinegar is observed.
CH3COO- + H+ → CH3COOH
The following test is performed with the aqueous salt solution.
(ii) Iron (III) Chloride Solution: Gives deep - red colouration
CH3COONa + FeCl3 → (CH3COO)3Fe + 3NaCl
Brown colour
6. Thiosulphates
(i) Dil Hydrochloric acid: Gives sulphur & sulphur di oxide
(ii) Silver nitrate solution: Gives white ppt. of silver thiosulphate.
S2O32-+ 2Ag+ → Ag2S2O3 ¯
The ppt. is unstable, turning dark on standing, when silver sulphide is formed.
Ag2S2O3¯ + H2O → Ag2S + H2SO4
(iii) Lead acetate or Lead nitrate solution: Gives white ppt.
S2O32-+ Pb2+ → PbS2O3 ¯
On boiling it turns black due to the formation of PbS.
PbS2O3 ¯ + H2O →PbS ¯ + 2H+ + SO42
7. Chloride (Cl-)
(i) Conc. H2SO4 : decomposes with the evolution of HCl.
Cl- + H2SO4 → HCl + HSO
Gas so produced
(1) Turns blue litmus paper red
(2) Gives white fumes of NH4Cl when a glass rod moistened with ammonia solution is brought near the mouth of test tube.
(ii) Silver nitrate solution: White, curdy ppt. of AgCl insoluble in water & in dil .nitric acid, but soluble in dilute ammonia solution.
(iii) Chromyl chloride test: When a salt containing chloride ion is heated with K2Cr2O7 and conc. H2SO4 orange red fumes of chromyl chloride (CrO2Cl2) are formed.
K2Cr2O7 + 4NaCl + 6H2SO4 → 2KHSO4 + 4NaHSO4 + 2CrO2Cl2 + 3H2O
orange – red fumes
Chlorides of mercury, owing to their slight ionization, do not respond to this test and only partial conversion to CrO2Cl2 occurs with the chlorides of lead, silver, antimony and tin.
When chromyl chloride vapours are passed into sodium hydroxide a yellow solution of sodium chromate is formed which when treated with lead acetate gives yellow ppt. of lead chromate.
8. Bromide (Br-)
(i) Conc. H2SO4: Gives reddish brown vapours of bromine accompanying the hydrogen bromide.
(ii) Manganese dioxide and conc. sulphuric acid: When a mix of solid bromide,
MnO2 and conc. H2SO4 is heated reddish brown vapours of bromine are evolved.
2KBr + MnO2 + 2H2SO4 → Br2 + K2SO4 + MnSO4 + 2H2O
The following tests are performed with the aqueous salt solution.
(iii) Silver nitrate solution: Pale yellow ppt. of silver bromide is obtained. This ppt. is sparingly soluble in dil but readily soluble in conc. ammonia solution and insoluble in dil. HNO3.
(iv) Lead acetate solution: White crystalline ppt. of lead bromide which is soluble in
boiling water.
9. Iodide (I-)
(i) Conc. H2SO4: Gives violet vapours of iodine
(ii) Silver nitrate solution: Yellow ppt. of silver iodide AgI, very slightly soluble in conc. ammonia solution and insoluble in dil nitric acid.
10. Nitrate (NO3- )
(i) Conc H2SO4: Gives reddish - brown vapours of nitrogen dioxide
4NO3- + 2H2SO4 → 4NO2 + 2SO42- + 2H2O + O2
(ii) Brown ring test: When freshly saturated solution of iron (II) sulphate is added to nitrate solution and conc. H2SO4 is poured slowly down the side of the test - tube, a brown ring is obtained.
2NO3- + 4H2SO4 + 6Fe2+ → 6Fe3+ +2NO + 4SO4–2 + 4H2O
Fe2+ + NO → [Fe(NO)]2+
On shaking and warming the mix, the brown colour disappears, nitric oxide is evolved and a yellow solution of Iron(III) ions remains.
11. Sulphate (SO42-)
(i) Barium chloride solution: White ppt. of barium sulphate BaSO4 insoluble in warm dil. hydrochloric acid and in dilute nitric acid, but moderately soluble in boiling, conc. hydrochloric acid.
(ii) Mercury (II) nitrate solution: Gives yellow ppt. of basic mercury (II) sulphate
12. Chromate CrO42 -and Dichromate (Cr2O)
(i) Barium chloride solution: Pale - yellow ppt. of barium chromate soluble in dilute mineral acids but insoluble in water and acetic acid.
CrO + Ba2+ → BaCrO4 ¯
Dichromate ion also gives the same ppt. but due to the formation of strong acid precipitation is partial.
Cr2O+ 2Ba2+ + H2O 2 BaCrO4 ¯ + 2H+
If sodium hydroxide or sodium acetate is added, precipitation becomes quantitative.
(ii) Silver Nitrate Solution: Brownish - red ppt. of silver chromate Ag2CrO4 which is soluble in dil. nitric acid & in ammonia solution, but insoluble in acetic acid.
A reddish brown ppt. of silver dichromate Ag2Cr2O7 is formed with a conc. solution of a dichromate.
13. Permanganate MnO
(i) Hydrogen peroxide : It decolourises acidified potassium permanganate solution
2MnO4- + 5H2O2 + 6H+ ¾¾→ 5O2 + 2Mn2+ + 8H2O.
(ii) Iron (II) sulphate, in the presence of sulphuric acid, reduces permanganate to
manganese (II). The solution becomes yellow because of the formation of iron (III) ions
MnO4- + 5Fe2+ + 8H+ → 5Fe3+ + Mn2+ + 4H2O
TEST FOR CATIONS
| Group | Group reagent | Ions | Colour & ppt. |
| Group I | dil HCl | Pb2+, Hg+, Ag+ | PbCl2, Hg2Cl2, AgCl - white |
| Group II Group II A Group II B | H2S in dil HCl | Hg2+, Cu2+, Bi3+, Cd2+ As3+, As5+, Sb3+, Sb5+, Sn2+, Sn4+ | Yellow - CdS,As2S3, As2S5 , SnS2 Black - HgS, CuS, PbS Orange - Sb2S3, Sb2S5 Brown - Bi2S3 ,SnS |
| Group III A | NH4OH in presence of NH4Cl | Fe3+, Al3+, Cr3+ | Fe(OH)3, Al(OH)3,Cr(OH)3 Brown White Green |
| GroupIII B | H2S in presence of NH3 & NH4Cl or (NH4)2S. | Ni2+, Co+2, Mn+2, Zn2+ | ZnS - white or grey, Black - CoS, NiS MnS - Buff (light pink) |
| Group IV | (NH4)2CO3 in presence of NH4Cl & NH4OH. | Ba+2, Sr2+, Ca+2 | BaCO3, SrCO3, CaCO3 - white |
| Group V | No common group reagent. | Mg+2, Na+, K+, NH4+ | ¾ |
1. Group I (Pb2+, Ag+, Hg+)
- PbCl2 gives a yellow ppt. with K2CrO4. The ppt. is insoluble in acetic acid but soluble in NaO
- PbCl2 + 2KI → PbI2 + 2KCl
(Yellow)
PbCl2 + 2KI (excess) → K2[PbI4]
AgCl is soluble in NH4OH forming a complex while Hg2Cl2 forms a black ppt. with NH4OH.
AgCl + 2NH4OH → Ag(NH3)2Cl + 2H2O
Hg2Cl2 + 2NH4OH → H2NHgCl + Hg + NH4Cl + 2H2O
Amino mercuric Chloride
2. Group II A (Hg2+, Cu2+, Bi3+, Cd2+)
(i) Hg+2 ions in solution, on addition of SnCl2, give a white precipitate turning black.
2Hg+2 + SnCl2 → Sn+4 + Hg2Cl2
White
Hg2Cl2 + SnCl2 → SnCl4 + 2Hg
Black
(ii) Cu+2 ions in solution give deep blue colour with excess of NH4OH
Cu+2 + 4NH4OH → [Cu(NH3)4 ]+2 + 4H2O
Deep blue in colour
Cu+2 ions give chocolate precipitate with K4Fe(CN)6.
2Cu+2 + K4Fe(CN)6 → Cu2[Fe(CN)6] + 4K+
(iii) Bi+3 ions in solution of HCl on addition of water give white cloudy precipitate.
BiCl3 + H2O → BiOCl + 2HCl
White ppt.
When treated with sodium stannite a black ppt. is obtained.
2BiCl3 + 3Na2SnO2 → 2Bi ¯ + 3Na2SnO3 + 6NaCl + 3H2O
black
(iv) Cd+2 ions in solution, with NaOH give a white precipitate.
Cd+2 + 2NaOH → Cd(OH)2 + 2Na+
white
With ammonium hydroxide, Cd2+ ions give a white precipitate which dissolves in excess.
Cd2+ + 4NH4OH → [Cd(NH3)4](OH)2 + 2H2O
3. Group II B (As3+, As5+, Sb3+, Sb5+, Sn3+, Sn4+)
- As+3 ions in solution give yellow precipitate with ammonium molybdate and HNO3 on heating.
H3AsO4 +12(NH4)2MoO4 +21HNO3 → (NH4)3 AsMo12O40+ 21NH4NO3 + 12H2O
Yellow ppt. - Sn2+ ions in solution as SnCl2 give white ppt. with HgCl2 ,which turns black on standing.
vii) Sb+3 ions in solution as SbCl3, on addition of water give white precipitate.
SbCl3 + H2O → SbOCl + 2HCI
White
4. Group III A (Al3+, Fe3+, Cr3+)
1. White precipitate of Al(OH)3 is soluble in NaOH
- Al(OH)3 + NaOH → NaAlO2 + 2H2O
2. Precipitate of Cr(OH)3 is soluble in NaOH + Br2 water and addition of BaCl2 to this solution gives yellow precipitate. Fe(OH)3 is insoluble in NaOH
3. Brown precipitate of Fe(OH)3 is dissolved in HCl and addition of KCNS to this solution gives blood red colour.
Also on addition of K4Fe(CN)6 to this solution, a prussian blue colour is obtained.
FeCl3 + 3K4Fe(CN)6 → Fe4[Fe(CN)6]3 + 12KCl
prussian blue colour
5. Group III B (Ni2+, Co2+, Mn2+, Zn+2)
(i) Ni+2 and Co+2 ions in solution, on addition of KHCO3 and Br2 water give apple green colour if Co+2 is present and black precipitate if Ni+2 is present.
CoCl2 + 6KHCO3 →K4[Co(CO3)3] + 2KCl + 3CO2 + 3H2O
2K4[Co(CO3)3] + 2KHCO3 + [O] → 2K3[Co(CO3)3] + 2K2CO3 + H2O
Apple green colour
NiCl2 + 2KHCO3 → NiCO3 + 2KCl + H2O + CO2
2NiCO3 + 4NaOH + [O] → Ni2O3 + 2Na2CO3 + 2H2O
Black ppt.
(ii) Zn+2 ions in solution give white precipitate with NaOH, which dissolve in excess of NaOH.
(iii) Mn+2 ions in solution give pink precipitate with NaOH turning black or brown on heating.
6. Group IV (Ba2+, Sr2+, Ca2+)
- Ba+2 ions in solution give:
Yellow precipitate with K2CrO4
Ba+2 + K2CrO4 → BaCrO4 + 2K+
YellowWhite precipitate with (NH4)2SO4
- Sr+2 ions give white precipitate with (NH4)2SO4 and (NH4)2C2O4
- Ca+2 ions give white precipitate with (NH4)2 C2O4 only.
7. Group V (NH4+, Na+, K+, Mg+2)
All ammonium salts on heating with alkali say NaOH give a colourless gas with a pungent
smell (NH3)
(A) Gas evolved gives white fumes with HCl
(B) Paper soaked in CuSO4 solution, is turned deep blue by NH3 due to complex formation
(C) With Hg2 (NO3)2 , a black colour is obtained
Hg2(NO3)2 + 2NH3 → Hg + Hg(NH2)NO3 ¯ + NH4NO3
black
(D) With Nesslers reagent (alkaline solution of potassium tetraiodomercurate(II) ), a brown ppt. is obtained
- Potassium salts give yellow ppt. with sodium cobalt nitrite
- Sodium salts give a heavy white ppt. with potassium dihydrogen antimonate
- Mg2+ gives white ppt. of magnesium hydroxide with sodium hydroxide
FAQs on Revision Notes: Principles Related to Practical Chemistry - NCERT Exemplar & Revision Notes for NEET
| 1. What are the main principles related to practical chemistry in the NEET exam? |  |
| 2. What are some common laboratory techniques that are important in practical chemistry for the NEET exam? |  |
| 3. Why is knowledge of safety procedures crucial in practical chemistry for the NEET exam? |  |
| 4. How important is the understanding of measurements and calculations in practical chemistry for the NEET exam? |  |
| 5. How does the interpretation of experimental data play a role in practical chemistry for the NEET exam? |  |





















