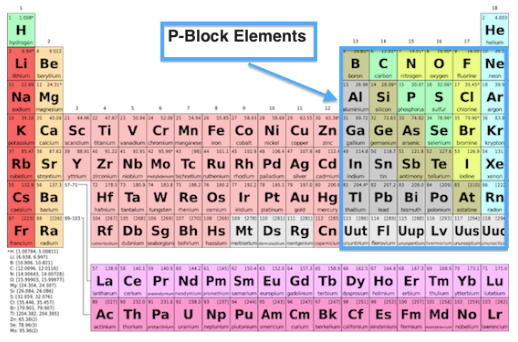
The p-Block Elements Class 11 Notes Chemistry
Boron Family (Group 13 Elements )
- Members: B, Al, Ga, In & Tl
- Melting Point: Decreases from B to Ga and then increases up to Tl.
- Ionization Energies: 1st <<< 2nd < 3rd
- Metallic Character: Increases from B to Tl. B is non-metal
Boron
Preparation of Boron:
- From Boric Acid: B2O3(s) + 3Mg(s) → 2B(s) +3 MgO(s)
- From Boron Trichloride
- (at 1270 k): 2BCl3+ 3H2 (g) → 2B(s) + 6HCl (g)
- (at 900 0C): 2BCl3(g) + 3Zn (s) → 2B(s) + 3 ZnCl2 (s)
- By electrolysis of fused mixture of boric anhydride (B2O3) and magnesium oxide (MgO) & Magnesium fluoride at 1100 0C
- 2 MgO- → 2Mg + O2(g)
- B2O3 + 3Mg → 2B + 3MgO
- By thermal decomposition of Boron hydrides & halides:
B2H6 (g) + Δ → 2B(s) + 3H2 (g)
Compounds of Boron:
Orthoboric acid (H3BO3)
Preparation of Orthoboric acid
- From borax : Na2B4O7 + H2SO4 + 5H2O → Na2SO4 + 4H3BO3
- From colemanite : Ca2B6O11 + 2SO2 + 11H2O → 2Ca(HSO3)2 + 6H3BO3
Properties of Orthoboric acid
- Action of Heat:
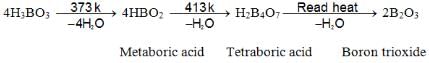
- Weak monobasic acidic behavior:
B(OH)3 ↔ H3BO3 ↔ H+ + H2O +
Thus on titration with NaOH, it gives sodium metaborate salt
H3BO3 + NaOH ↔ NaBO2 + 2H2O
- Reaction with Metaloxide:

- Reaction with Ammonium boro fluoride:
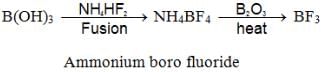
Borax (sodium tetraborate) Na2B4O7. 10H2O
Preparation from Boric Acid
4H3BO3 + Na2CO3 --> Na2B4O7 + 6H2O + CO2
Properties of Borax
- Basic Nature:-
Aqueous solution of borax is alkaline in nature due to its hydrolysis
Na2B4O7 + 3H2O → NaBO2 + 3H3BO3
NaBO2 + 2H2O → NaOH + H3BO3
- Action of heat:
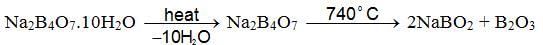
Diborabe( B2H6)
Preparation of Diborane:
Reduction of Boron Trifluoride:
BF3 + 3LiAlH4 → 2B2H6 + 3 LiAl F4
From NaBH4:
2NaBH4 + H2SO4 → B2H6 + 2H2 + Na2SO4
2NaBH4 + H3PO4 → B2H6 + 2H2 + NaH2PO4
Properties of Diborane:
- Reaction with water: B2H6 + H2O -->2H3BO3 + 6H2
- Combustion: B2H6 +2O2 --? B2O3 + 3H2O ΔH = -2615 kJ/mol
Compounds of Aluminium:
Aluminium Oxide or Alumina (Al2O3)
2Al(OH)3 +Heat → Al2O3 + 2H2O
2Al(SO4)3 +Heat → Al2O3 + 2SO3
(NH4)2Al2(SO4)3·24H2O --> 2NH3 +Al2O3 + 4SO3 + 25 H2O
Aluminum Chloride AlCl3:
Structure of Aluminium Chloride: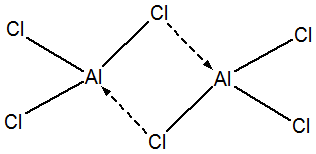
Properties of Aluminium Chloride
- White, hygroscopic solid
- Sublimes at 183 0C
- Forms addition compounds with NH3, PH3, COCl2 etc.
- Hydrolysis: AlCl3 + 3H2O --> Al(OH)3 + 3HCl + 3H2O
- Action of Heat: 2AlCl3 .6H2O --> 2Al(OH)3 à Al2O3+ 6HCl + 3H2O
Carbon Family (Group 14 Elements):
- Members: C, Si, Ge, Sn, & Pb
- Ionization Energies: Decreases from C to Sn and then increases up to Pb.
- Metallic Character: C and Si are non metals, Ge is metalloid and Sn and Pb are metals
- Catenation: C and Si show a tendency to combine with its own atoms to form long chain polymers
Compounds of Carbon:
Carbon Monoxide
Preparation of Carbon Monoxide
- By heating carbon in limited supply of oxygen: C + 1/2O2 --> CO.
- By heating oxides of heavy metals e.g. iron, zinc etc with carbon.
- Fe2O3 + 3C → 2Fe + 3CO
- ZnO + C → Zn + CO
- By passing steam over hot coke: C + H2O → CO + H2 (water gas)
- By passing air over hot coke: 2C + O2 + 4N2 → 2CO + 4N2 (Producer gas)
Properties of Carbon Monoxide:
- A powerful reducing agent : Fe2O3 + 3CO → 2Fe + 3CO2
CuO + CO → Cu + CO2 - Burns in air to give heat and carbon dioxide: CO + 1/2O2 → CO2 + heat.
Tests For Carbon Monoxide:
- Burns with blue flame
- Turns the filter paper soaked in platinum or palladium chloride to pink or green.
Carbon di-oxide
Preparation of Carbon di-oxide
- By action of acids on carbonates: CaCO3 + 2HCl → CaCl2 + H2O + CO2
- By combustion of carbon: C + O2 → CO2
Properties of Carbon di-oxide
- It turns lime water milky Ca(OH)2 + CO2 → CaCO3 ¯ + H2O,
- Milkiness disappears when CO2 is passed in excess
CaCO3 + H2O + CO2 → Ca(HCO3)2 - Solid carbon dioxide or dry ice is obtained by cooling CO2 under pressure. It passes from the soild state straight to gaseous state without liquefying (hence dry ice).
Carbides:
- Salt like Carbides : These are the ionic salts containing either C22- (acetylide ion) or C4- (methanide ion)e.g. CaC2, Al4C3, Be2C.
- Covalent Carbides : These are the carbides of non-metals such as silicon and boron. In such carbides, the atoms of two elements are bonded to each other through covalent bonds. SiC also known as Carborundum.
- Interstitial Carbides : They are formed by transition elements and consist of metallic lattices with carbon atoms in the interstices. e.g. tungsten carbide WC, vanadium carbide VC.
Compounds of Silicon:
Sodium Silicate (Na2SiO3):
Prepared by fusing soda ash with pure sand at high temperature:
Na2CO3+ SiO3 → Na2SiO3 +CO2
Silicones:
Silicon polymers containing Si – O – Si linkages formed by the hydrolysis of alkyl or aryl substituted chlorosilanes and their subsequent polymerisation.
Silicates:
Salts of silicic acid, H4SiO4 comprised of SiO44- units having tetrahedral structure formed as result of sp3 hybridization.
Nitrogen Family (Group 15 Elements)
- Members: N, P, As, Sb & Bi
- Atomic Radii: Increases down the group. Only a small increases from As to Bi.
- Oxidation state: +3, +4 & +5.Stability of +3 oxidation state increases down the group.
- Ionization energy: Increases from N to Bi.
Nitrogen
Preparation of Nitrogen:
- 3CuO + 2NH3 + Heat --> N2 + Cu + 3H2O
- CaOCl2 + 2NH3 + Heat --> CaCl2+ 3H2O + N2
- NH4NO2 +Heat --> 3H2O + N2 +Cr2O3
Properties of Dinitrogen:
- Formation of Nitrides (with Li, Mg, Ca & Al): Ca + N2 +Heat → Ca3N2
- Oxidation: N2 + O2 → 2NO
- Reaction with carbide (at 1273 K): CaC2 + N2 → CaCN2 + C
Oxides of Nitrogen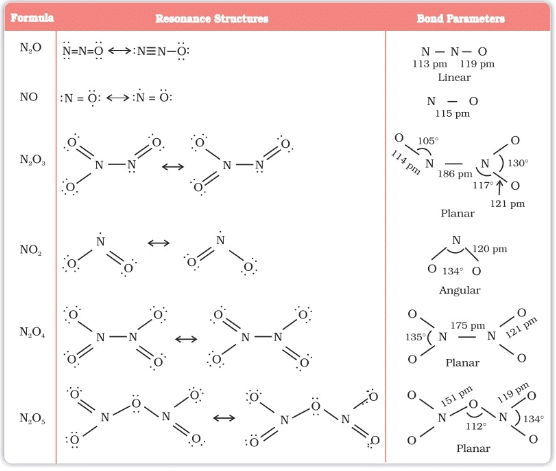
Oxy -Acids of Nitrogen :
Oxy Acids | Name of oxy – acid |
1. H2N2O2 | Hyponitrous acid |
2. H2 NO2 | Hydronitrous acid |
3. HNO2 | Nitrous acid |
4. HNO3 | Nitric acid |
5. HNO4 | Per nitric acid |
Ammonia (NH3):
Preparation of Ammonia:
- By heating an ammonium salt with a strong alkali ;NH4Cl + NaOH --> NH3 + NaCl + H2O
- By the hydrolysis of magnesium nitride: Mg3N2 + 6H2O --> 3Mg(OH)2 + 2NH3.
- Haber's process : N2(g) + 3H2(g) --> 2NH3(g).
Properties of Ammonia:
- Basic nature : Its aq. solution is basic in nature and turns red litmus blue.
NH3 + H2O ↔ NH4+ + OH- - Reaction with halogens :
- 8NH3 + 3Cl2 --> 6NH4Cl + N2
- NH3 + 3Cl2 (in excess) → NCl3 + 3HCl
- 8NH3 + 3Br2 → 6NH4Br + N2
- NH3 + 3Br2 (in excess) → NBr3 + 3HBr
- 2NH3 + 3I2 → NH3.NI3 + 3HI
- 8NH3.NI3 → 6NH4I + 9I2 + 6N2
- Complex formation :
- Ag+ + NH3 → [Ag(NH3)2]+
- Cu2+ + 4NH3 → [Cu(NH3)4]2+
- Cd2+ + 4NH3 → [Cd(NH3)4]2+
Precipitation of heavy metal ions from the aq. solution of their salts :
- FeCl3 + 3NH4OH → Fe(OH)3 + 3NH4Cl
Brown ppt. - AlCl3 + 3NH4OH → Al(OH)3 + 3NH4Cl
White ppt. - CrCl3 + 3NH4OH → Cr(OH)3 + 3NH4Cl
Green ppt.
Phosphorus:
Allotropy of Phosphorus:
a) White phosphorus: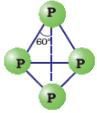
- Translucent white waxy solid
- Extremely reactive
- Poisonous and insoluble in water
b) Red Phosphorus:
- Formed by heating white phosphorus in absence of air.
- Does not burn spontaneously at room temperature.
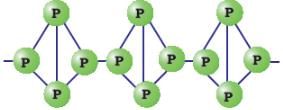
c) Black Phosphorus: Formed by further heating of red phosphorus.
Compounds of Phosphorus:
a) Phosphine, PH3:
Preparation of Phosphine
- Ca3P2 + 6H2O → 2 PH3 + 3 Ca(OH)2
- 4H3PO3 +Heat → PH3+ 3 H3PO4
- PH4I +KOH → PH3+KI + H2O
- P4 + 3KOH + 3H2O → PH3 +3KH3PO2
Properties of Phosphine:
- Formation of Phosphonic Iodide: PH3 + HI à PH4I
- Combustion: PH3 + 2O2 à H3PO4
b) Phosphorous Halides:
Preparation:
- P4+ 6Cl2 → 4PCl3
- P4+ 10Cl2 → 4PCl5
- P4+ 8SOCl2 → 4PCl3 + 4SO2+ 2S2Cl2
- P4+ 10SOCl2 → 4PCl5 + 10SO2
Properties:
- PCl3 + 3H2O → H3PO3 + 3HCl
- PCl5 + 4H2O → POCl3 à H3PO4 +5HCl
- PCl3 + 3CH3COOH → 3 CH3COCl +H3PO3
- PCl5 + CH3COOH → CH3COCl + POCl3+ HCl
- 2Ag + PCl5 → 2AgCl + PCl3
- 2Sn + PCl5 → SnCl4 + 2PCl3
- PCl5 + Heat → PCl3 + Cl2
C) Oxides of Phosphorus: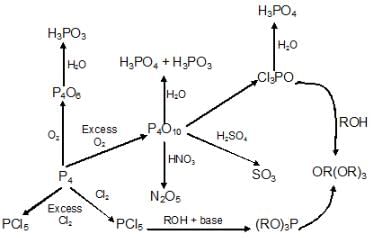
d) Oxy – Acids of Phosphorus:
Oxo acid | Name |
H3PO2 | Hypophosphorus acid |
H3PO3 | Phosphorus acid |
H4P2O6 | Hypophosphoric acid |
H3PO4 | Orthophosphoric acid |
H4P2O7 | Pyrophosphoric acid |
HPO3 | Metaphosphoric acid |
Oxygen Family (Group 16 Elements) :
Sr. No. | Property | Oxygen | Sulfur | Selenium | Tellurium | Polonium |
1. | Configuration | [He]2s22p4 | [Ne]3s23p4 | [Ar]4s24p4 | [Kr]5s25p4 | [Xe]6s26p4 |
2. | Common oxidation state | -2 | -2, +4, +6 | +4, +6 | +4, +6 | |
3. | Atomic radius (pm) | 66 | 104 | 116 | 143 | 167 |
4. | First ionization energy (KJ/mol) | 1314 | 1000 | 941 | 869 | 812 |
5. | Electronegativity | 3.5 | 2.5 | 2.4 | 2.1 | 2.0 |
Chemical Properties of Group 16:
Formation of volatile Hydrides:
Formation of Halides:
Formation of Oxide:
a) All elements (except Se) forms monoxide.
b) All elements form dioxide with formula MO2, SO2 is a gas, SeO2 is volatile solid. While TeO2 and PoO2 are non – volatile crystalline solids.
c) Ozone: It is unstable and easily decomposes into oxygen. It acts as a strong oxidising agent due to the case with which it can liberate nascent oxygen.
Oxyacids:
Sulphur | Selenium | Tellurium |
Sulphurous acid H2SO3. Sulphuric acid H2SO4 Peroxomonosulphuric acid H2SO5(Caro’s acid) Peroxodisulphuric acid H2S2O8 (Marshell’s acid) Thio sulphuric acid H2S2O3 Dithiconic acid H2S2O6 Pyrosulphuric acid H2S2O7 | Selenious acid H2SeO3 Selnenic acid H2SeO4 | Tellurous acid H2TeO3. Telluric acid H2TeO4. |
Allotropes of Sulphur :
Rhombic sulphur:
- It has bright yellow colour.
- It is insoluble in water and carbon disulphide. Its density is 2.07 gm cm-3 and exists as S8 molecules. The 8 sulphur atoms in S8 molecule forms a puckered ring.
Monoclinic Sulphur :
- Stable only above 369 K. It is dull yellow coloured solid, also called b - sulphur. It is soluble in CS2 but insoluble in H2O.
- It slowly changes into rhombic sulphur. It also exist as S8 molecules which have puckered ring structure. It however, differs from the rhombic sulphur in the symmetry of the crystals
Plastic Sulphur:
- It is obtained by pouring molten sulphur to cold water.
- It is amorphous form of sulphur.
- It is insoluble in water as well as CS2.
Sulphuric Acid:
- Due to strong affinity for water, H2SO4 acts as a powerful dehydrating agent.
- Concentrated H2SO4 reacts with sugar, wood, paper etc to form black mass of carbon. This phenomenon is called charring.
- It is moderately strong oxidizing agent.
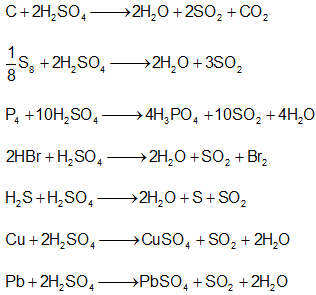
- Decomposes carbonates, bicarbonates, sulphides, sulphites, thiosulphates and nitrites at room temperatures.
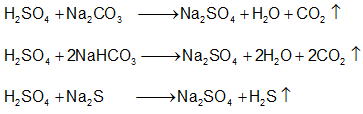
- Salts like chlorides, fluorides, nitrates, acetates, oxalates are decomposed by hot conc. H2SO4 liberating their corresponding acids.
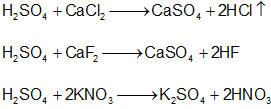
Halogen Family ( Group 17 Elements)
Inter halogen compounds:
Type XX’1 (n = 1) (with linear shape) | Type XX’3 (n = 3) (with T-shape) | XX’5 (n = 5) (with square pyramidal shape) | XX’7 (n = 7) with pentagonal bipyramidal shape) |
CIF | ClF3 | ClF5 | |
BrF BrCl | BrF3 | BrF5 | |
ICl, IBr, IF | ICl3, IF3 | IF5 | IF7 |
Hydrogen Halides:
Properties of Hydrogen Halides:
- All the three acids are reducing agents HCl is not attacked by H2SO4.
- 2HBr + H2SO4 → 2H2O + SO2 + Br2
- 2HI + H2SO4 → 2H2O + SO2 + I2
- All the three react with KMnO4 and K2Cr2O7
- 2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
- K2Cr2O7 + 14HBr → 2KBr + 2CrBr3 + 7H2O + 3Br2
- Other reactions are similar.
- Dipole moment : HI < HBr < HCl < HF
- Bond length: HF < HCl < HBr < HI
- Bond strength: HI < HBr < HCl < HF
- Thermal stability: HI < HBr < HCl < HF
- Acid strength: HF < HCl < HBr < BI
- Reducing power: HF < HCl < HBr < HI
Pseudohalide ions and pseudohalogens:
Ions which consist of two or more atoms of which at least one is nitrogen and have properties similar to those of halide ions are called pseudohalide ions. Some of these pseudohalide ions can be oxidised to form covalent dimers comparable to halogens (X2). Such covalent dimers of pseudohalide ions are called pseudohalogens.
The best known psuedohalide ion is CN–
Pseudohalide ions | Name |
CN– | Cyanide ion |
OCN– | Cyanate ion |
SCN– | Thiocyante ion |
SeCN– | Selenocyanate ion |
NCN2– | Cyanamide ion |
N3– | Azide ion |
OMC– | Fulminate ion |
Pseudohalogen
- (CN)2 cyanogen
- (SCM)2 thiocyanogen
Some important stable compound of Xenon
- XeO3 Pyramidal
- XeO4 Tetrahedral
- XeOF4 Square pyramidal
- XeO2F2 Distorted octahedral
First rare gas compound discovered was Xe+ (PtF6]– by Bartlett.
Oxyacids of Chlorine
Formula | Name | Corresponding Salt |
HOCl | Hypochlorous acid | Hypochlorites |
HClO2 | Chlorous acid | Chlorites |
HClO3 | Chloric acid | Chlorates |
HClO4 | Perchloric acid | Perchlorates |
Acidic Character: Acidic character of the same halogen increases with the increase in oxidation number of the halogen: HClO4> HClO3 > HClO2 > HOCl
Preparation
HOCl :
- Ca(OCl)2 + 2HNO3 → Ca(NO3)2 + 2HOCl
HClO2 :
- BaO2 + 2ClO2 → Ba(ClO2)2 (liquid) + O2
- Ba(ClO2)2 + H2SO4(dil.) → BaSO4 ¯ + 2HClO2
HClO3 :
- 6Ba(OH)2 + 6Cl2 → 5BaCl2 + Ba(ClO3)2 + 6H2O
- Ba(ClO3)2 + H2SO4(dil.) → BaSO4 ¯ + 2HClO3
HClO4 :
- KClO4 + H2SO4 → KHSO4 + HClO4
- 3HClO3 → HClO4 + 2ClO2 + H2O
The Noble Gases (Group 18 Elements):
The noble gases are inert in nature. They do not participate in the reactions easily because they have
- stable electronic configuration i.e. complete octet.
- high ionization energies.
- low electron affinity.
Compounds of Xenon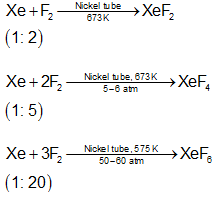
Molecule | Total electron pairs (BP + LP) | Hybridisation | Shape |
XeF2 | 5 | Sp3d | Linear |
XeF4 | 6 | Sp3d2 | Square planar |
XeF6 | 7 | sp3d3 | Distorted octahedral |
Uses of Nobles gas
The noble gases are used in following ways:
(A) Helium
- It is used to fill airships and observation balloons.
- In the oxygen mixture of deep sea divers.
- In treatment of asthma.
- Used in inflating aeroplane tyres.
- Used to provide inert atmosphere in melting and welding of easily oxidizable metals.
(B) Neon
- It is used for filling discharge tubes, which have different characteristic colours and are used in advertising purposes.
- Also used in beacon lights for safety of air navigators as the light possesses fog and stram perpetrating power.
(C) Argon
Along with nitrogen it is used in gas – filled electric lamps because argon is more inert than nitrogen.
FAQs on The p-Block Elements Class 11 Notes Chemistry
| 1. What are p-block elements and where are they located in the periodic table? |  |
| 2. How many valence electrons do p-block elements typically have? |  |
| 3. What are some common properties of p-block elements? |  |
| 4. What are the uses of p-block elements in everyday life? |  |
| 5. How do p-block elements form compounds with other elements? |  |

















