Selection Rules for Electronic Spectra of Transition Metal Complexes | Chemistry Optional Notes for UPSC PDF Download
Selection Rules
The Selection Rules governing transitions between electronic energy levels of transition metal complexes are:
- ΔS = 0 The Spin Rule
- Δl = +/- 1 The Orbital Rule (or Laporte)
The first rule says that allowed transitions must involve the promotion of electrons without a change in their spin. The second rule says that if the molecule has a center of symmetry, transitions within a given set of p or d orbitals (i.e. those which only involve a redistribution of electrons within a given subshell) are forbidden.
Relaxation of these rules can occur through:
- Spin-Orbit coupling: this gives rise to weak spin forbidden bands
- Vibronic coupling: an octahedral complex may have allowed vibrations where the molecule is asymmetric. Absorption of light at that moment is then possible.
- Mixing: π-acceptor and π-donor ligands can mix with the d-orbitals so transitions are no longer purely d-d.
Transition Types
- Charge transfer, either ligand to metal or metal to ligand. These are often extremely intense and are generally found in the UV but they may have a tail into the visible.
- d-d, these can occur in both the UV and visible region but since they are forbidden transitions have small intensities.
Expected intensities of electronic transitions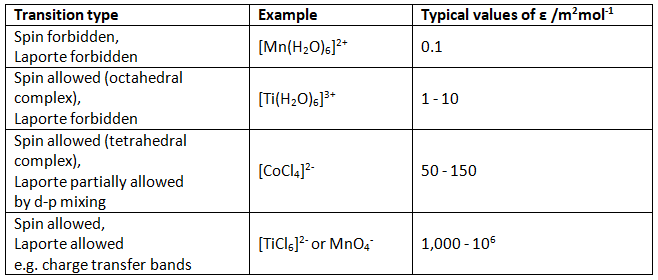
Expected Values
The expected values should be compared to the following rough guide.
- For M2+ complexes, expect Δ = 7,500 - 12,500 cm-1 or λ = 800 - 1,350 nm.
- For M3+ complexes, expect Δ= 14,000 - 25,000 cm-1 or λ = 400 - 720 nm.
For a typical spin-allowed, but Laporte (orbitally) forbidden transition in an octahedral complex, expect ε < 10 m2mol-1. Extinction coefficients for tetrahedral complexes are expected to be around 50-100 times larger than for octrahedral complexes. B for first-row transition metal free ions is around 1,000 cm-1. Depending on the position of the ligand in the nephelauxetic series, this can be reduced to as low as 60% in the complex.
FAQs on Selection Rules for Electronic Spectra of Transition Metal Complexes - Chemistry Optional Notes for UPSC
| 1. What are selection rules for electronic spectra of transition metal complexes? |  |
| 2. What factors determine the transition types in electronic spectra of transition metal complexes? |  |
| 3. What are some commonly observed transitions in electronic spectra of transition metal complexes? |  |
| 4. How do selection rules affect the absorption or emission of light by transition metal complexes? |  |
| 5. How do selection rules contribute to the understanding of the electronic structure of transition metal complexes? |  |





















