Morrison & Boyd Test: Alcohols, Phenols & Ethers | Chemistry Class 12 - NEET PDF Download
Q.1 What is rectified spirit ?
Answer: A mixture of 95% ethyl alcohol and 5% water is called rectified spirit.
Q.2 Name the alcohol used for polishing wooden furniture.
Answer: Ordinary spirit.
Q.3 Which type of alcohol is formed by hydroboration oxidation of unsymmetrical alkene?
Answer: In case of hydro oration oxidation of alkene the alcohol formed is (primary alcohol) opposite to Markovnikov's rule.
Q.4 Among acids and esters which can be reduced to alcohol easily by catalytic hydrogenation?
Answer: Esters can be easily reduced to alcohols.
Q.5 Name the product formed by reaction of Grignard's reagent with an aldehyde followed by hydrolysis.
Answer: Alcohol (1°alcohol with methanal and 2° alcohol with other aldehydes).
Q.6 Which one has higher boiling point propane or ethanol?
Answer: Ethanol has higher boiling point than propane.
Q.7 Alcohols act as weak acid. Why?
Answer: Due to polar nature of O - H bond alcohol acts as weak acid.
Q.8 Which type of alcohol does not react with Lucas reagent at room temperature?
Answer: 1° alcohol does not react with Lucas reagent at room temperature.
Q.9 What is the rate of reactivity of 1°, 2° and 3° alcohols with hydrogen halide?
Answer: 3° > 2° > 1°. (Reactivity of hydrogen halide)
Q.10 What is the ease of dehydration of 1 °, 2° and 3° alcohols?
Answer: 3° > 2° > 1°. (Ease of dehydration of alcohol)
Q.11 What is wood spirit?
Answer: Methanol is also known as wood spirit.
Q.12 Which enzyme catalyses conversion of sucrose to ethanol?
Answer: Invertase followed by zymase.
Q.13 What is the main use of ethanol?
Answer: Solvent in paint industry.
Q.14 Explain denaturation.
Answer: The process of making alcohol unfit for drinking is called denaturation.
Q.15 Which compound is mixed in ethanol for denaturation?
Answer: Copper sulphate and pyridine.
Q.16 Which alcohol on oxidation gives aldehyde?
Answer: 1° alcohol on oxidiation gives aldehyde.
Q.17 Which alcohol on oxidation gives ketone?
Answer: 2° alcohol on oxidation gives ketone.
Q.18 What are phenols?
Answer: When the hydrogen atom is replaced by -OH group in benzene ring the compound obtained is called phenol.
Q.19 What is carbolic acid?
Answer: Phenol is also called carbolic acid.
Q.20 Phenols are acidic. Why?
Answer: Due to presence of polar -OH group phenol behaves as acid and phenoxide ion is more stable.
Q.21 What is the hybridisation of oxygen atom in ether?
Answer: sp3.
Q.22 Boiling points of ethers are much lower than that of alcohols. Why?
Answer: Ethers do not form H-bonding due to which they have lower boiling points than corresponding alcohols.
Q.23 Boiling points of alcohols are higher than correspond in hydrocarbons, ethers and alkyl halides. Why?
Answer: The high boiling points of alcohols are mainly due to the presence of intermolecular hydrogen bonding in them which is lacking in ethers, hydrocarbons and alkyl halides.
Q.24 Boiling points of alcohols decrease with increase in branching of carbon chains. Why?
Answer: As the branching increases surface area decreases and hence magnitude of Van der Waals force also decreases so, boiling point decreases.
Q.25 Boiling points of ethanol is much higher than methoxy methane. Why?
Answer: Ethanol has high boiling point than methoxy methane because alcohols are soluble in water due to formation of H-bonding with water molecule.
Q.26 Solubility of alcohols in water decreases with increasing size of alkyl group. Why?
Answer: Alkyl groups are hydrophobic in nature. So, as size of alkyl group increases, its solubility in water decreases.
Q.27 What is the chief use of methanol?
Answer: Methanol is chiefly used for the preparation of formaldehyde.
Q.28 Which alcohol is highly poisonous?
Answer: Methanol is highly poisonous.
Q.29 What is the advantage of using PCC for the oxidation of alcohols?
Answer: Oxidation stops at aldehyde level.
Q.30 Phenols are more reactive than benzene towards electrophilic substitution reaction. Why?
Answer: Resonating structures show that benzene ring acquires -ve charge which implies that there is a greater electron cloud. Therefore, an electrophile readily attacks at o/p position of phenol than in benzene.
Q.31 Anisole undergoes bromination with bromine and ethanoic acid even in absence of iron bromide catalyst. Why?
Answer: Anisole undergoes bromination with bromine and ethanoic acid even in absence of iron (III) bromide catalyst due to activation of benzene ring by the methoxy group.
Q.32 What are the conditions for preparation of ethers from alcohols?
Answer: Conditions for the preparation of ethers from alcohols are : (i) only 1° alcohol should be taken. (ii) alkyl should be unhindered. (iii) temperature should be kept low.
Q.33 Why is it difficult to prepare ethyl methyl ether by dehydration of alcohols?
Answer: For the preparation of ethyl methyl ether two different types of alcohols are needed. As a result a mixture products is formed which is difficult to separate.
Q.34 What are the advantages of Williamson's method.
Answer: (i) Both symmetrical and unsymmetrical ethers can be prepared by this method. (ii) 2° and 3° ethers can also be prepared by this method
Q.35 Why is Williamson's synthesis not applicable to tertiary halides?
Answer: In case of secondary or tertiary alkyl halides elimination competes over substitution and product formed is alkene.
Q.36 Tertiary alcohols are easiest to dehydrate, why?
Answer: During the process of dehydration the intermediate formed is carbocation. We know that 3°-carbocation is most stable carbocation. So, it is easiest to dehydrate 3°-alcohol.
Q.37 How is phenol obtained from cumene?
Answer: In alcohols, -OH group is attached to electron releasing alkyl group which decreases polarity of O-H bond while in phenols -OH group is attached to electron withdrawing phenyl group which increases polarity of O - H bond.
Q.38 What is denatured alcohol?
Answer: Ethanol, when mixed with copper sulphate (to give it colour) and pyridine (a foul smelling liquid) is called denatured alcohol. It affects judgements and lowers inhibition and also cause nausea and loss of consciousness. Ethanol oxidises to ethanoic acid which is the cause of diseases.
Q.39 Why is fermentation carried out in absence of air?
Answer: If air gets into fermentation mixture, it oxidises ethanol to ethanoic acid which destroy the taste of alcoholic drinks.
Q.40 What is the order of reactivity of alcohols in case of esterification?
Answer: Rate of esterification: 1° > 2° > 3° alcohol.
Q.41 Alcohols can act both as acids and bases. Explain.
Answer: Alcohols can act as acids in strongly basic medium by releasing H+ion. Similarly, they can act as Lewis bases in the acidic medium.
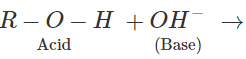
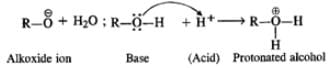
Q.42 Sodium metal cannot be used for drying alcohols. Assign reason.
Answer: Sodium metal reacts with alcohol to evolve hydrogen gas. Moreover, it also catches fire in hatr. Therefore, it can not be used for drying alcohol. 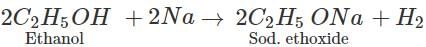
Q.43 Ethyl alcohol and dimethyl ether are isomeric but alcohol is a liquid at room temperature while ether is a gas. Explain.
Answer: Ethyl alcohol molecules are associated due to intermolecular hydrogen bonding but the same is not present in the molecules of dimethyl ether. As a result of association, ethyl alcohol is a liquid at room temperature while dimethyl ether is a gas.
Q.44 At room temperature tertiary alcohols form white turbidity very fast with Lucas reagent while primary alcohols do not. Give reason.
Answer: A carbocation intermediate is formed when HCl(g) reacts with an alcohol in the presence of anhydrous ZnCl2(dehydrating agent). Since tertiary carbocation is very stable while primary carbocation is rather unstable therefore, tertiary alcohols react very fast with Lucas reagent to form white turbidity immediately while the primary alcohols do not react at room temperature.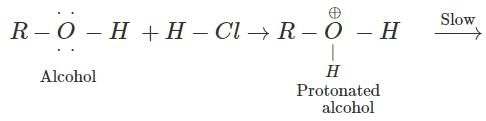

Q.45 Predict the product of the reaction between HBr and but-2-en-l-ol.
Answer:
CH3−CH 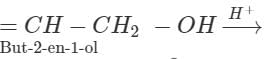 CH3−CH=CH−CH2−
CH3−CH=CH−CH2− 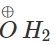
Q.46 Hydration of 3-phenylbut-l-ene in dilute H2SO4 forms 2-phenylbutan-2-ol and not 3-phenylbutan-2-ol. Why?
Answer: The secondary carbocation initially formed as a result of hydration changes to a more stable tertiary carbocation by H− ion shift. Therefore, the final product is different from the expected product.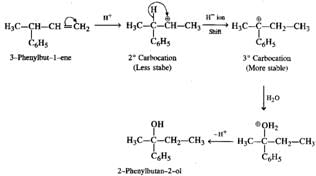
Q.47 On reacting tertiary butyl alcohol and normal butyl alcohol separately with a few drops of dilute KMnO4,purple colour disappears and a brown precipitate is formed only in one case. Which of the two alcohols will respond to the reaction?
Answer: Only n-butyl alcohol is oxidised under the reaction conditions while tertiary butyl alcohol fails to react.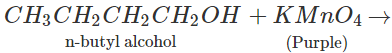
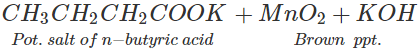
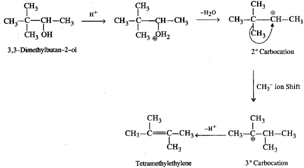
Q.48 3, 3-Dimethyl butan-2-ol loses a molecule of water in the presence of concentrated sulphuric acid to give tetramethylethylene as the major product. Suggest a suitable mechanism.
Answer:
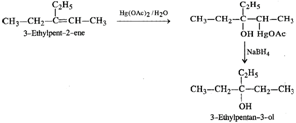
Q.49 What is the structure of the major product when 3-Ethylpent-2-ene is reacted with Hg(OAc)2/H2O;NaBH4?
Answer: The reaction is known as oxymercuration-demercuration and proceeds as follows :
Q.50 p-nitrophenol is a stronger acid than phenol while p-cresol is a weaker acid. Discuss.
Answer: The −NO2 group is an electron withdrawing group. When present at the para position in the ring, it decreases the electron density on the oxygen atom of O?H group due to conjugation and the release of H+ ion becomes easier than in phenol. On the other hand, −CH3 group is an electron releasing group. It increases the electron density on the oxygen atom and the release of H+ ion becomes difficult compared to phenol. Therefore, p-nitrophenol is a stronger acid than phenol while p-cresol is a weaker acid. For further details, consult section 12.16 (Chemical properties of phenols).
Q.51 How do you account for the fact that unlike phenol, 2,4-dinitrophenol is soluble in aqueous sodium carbonate solution?
Answer: The presence of two electron withdrawing −NO2 groups in the ring at the ortho and para positions makes 2,4-dinitrophenol a stronger acid than phenol. It therefore, reacts with aqueous sodium cartonate to form corresponding sodium salt which is soluble. But phenol being a weaker acid does not react with aqueous sodium carbonate. For more details, consult section 12.16.
Q.52 Alcohols react with halogen acids as well as phosphorus halides to form haloalkanes but phenols do not form haloarenes. Explain.
Answer: In phenols, the CO bond has a partial double bond character due to resonance. Therefore, it is not easily cleaved by the X− ion of halogen acids or phosphorus halides. But the bond is a pure single bond in alcohols and cleavage is therefore, possible. Thus, alcohols easily form haloalkanes while phenols do not form haloarenes.
Q.53 The order of reactivity of alcohols in the esterification reaction is : primary > secondary > tertiary. Justify.
Answer: In the esterification reaction, OH bond of alcohol cleaves. The presence of alkyl group with +I effect makes the cleavage of the bond difficult and the alcohol becomes less reactive towards esterification. Greater the number of alkyl groups present, lesser will be the reactivity of alcohol. The relative order of reactivity is, therefore, justified. For more details, consult section 12.8.
Q.54 Dehydration of alcohol to form an alkene is always carried out with concentrated H2SO4 and not with concentrated HCl or HNO3. Explain.
Answer: Under the acidic conditions, alcohol is initially protonated and then loses H2O to form a carbocation. If HCl is used, then Cl− ion being a strong nucleophile will result in nucleophilic substitution to form alkyl chloride. However,HSO4- ion released by H2SO4 is a very weak nucleophile and cannot participate in the nucleophilic substitution. It will rather act as a base and eliminate a proton to form alkene as the product as follows :
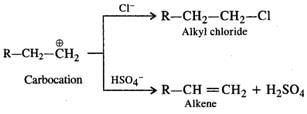
Concentrated HNO3 is a powerful oxidising agent. It will cause oxidation of alcohol to aldehyde and then to acid. Thus, out of the mineral acids listed, dehydration is carried by concentrated H2SO4. Even phosphoric acid can be used.
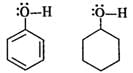
Q.55 Phenol is acidic while cyclohexanol is neutral. Justify.
Answer: In phenol, the oxygen atom of OH group becomes electron deficient due to the conjugation of electron pairs on it with the pi electron pairs of ring. Therefore, H+ can be released. However, in cyclohexanol, the electron pairs on the oxygen atom are not involved in any conjugation with the ring since it has no pi electron pairs. Therefore, the release of H+ion is quite difficult.
Q.56 Phenol has a smaller dipole moment than methanol. Discuss.
Answer: 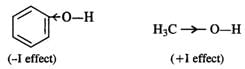
Phenyl group has −I (inductive effect) and it tends to decrease the electron density on the oxygen atom. As a result the polarity or dipole moment of OH bond is less than in methyl alcohol in which methyl group has +I effect.
Q.57 o-nitrophenol is more acidic than o-methoxyphenol. Explain.
Answer: Nitro group is an electron withdrawing group. It tends to withdraw the electrons from the ring as well as from the oxygen atom of OH bond. This facilitates the release of proton. It is therefore, a stronger acid than o-methoxyphenolin which the methoxy group is an electron. For more details, consult section 12.16 (chemical properties of phenols).
Q.58 The compound C4H10O is produced on reaction of an alkane with H2SO4/H2O which is not resolvable into optical isomers. Identify the compound.
Answer: The molecular formula of the compound and the reaction conditions suggests that the compound is an alcohol and not an ether. Since it is not resolvable, it is symmetrical and isotertiary butyl alcohol. The corresponding alkane is isobutane. The alcohol has been formed as follows :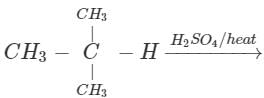
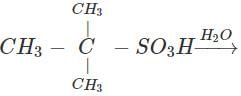
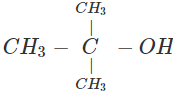
Q.59 Which of the following is the most reactive towards attack by an electrophile?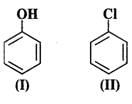
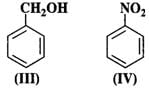
Answer: An electrophile will always prefer to attack centres of high electron density in the ring. Both nitro (−NO2) and chloro (?Cl) groups are electron withdrawing groups. In benzyl alcohol (III), the OH group is not directly attached to the ring and is not in a position to activate it. Phenol (I) is the most reactive because phenolic group activates the ring towards electrophilic attack both at the ortho and para positions. For more details, consult section 12.16 (Chemical properties of phenols).
Q.60 What is the major product of the hydration of following alkenes?
(i) 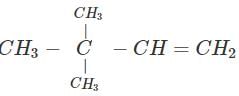 (ii)
(ii) 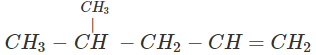
Answer: 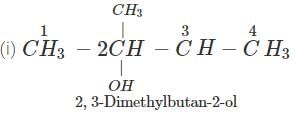
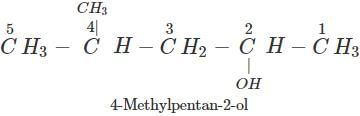
Q.61 Why is cyclohexanol more soluble in water than hexan-1-ol?
Answer: In cyclohexanol, group R(−C6H11) is more compact than the group (−C6H13) in hexan-1-ol which is a straight alcohol. Due to smaller size, the cyclohexyl group is more exposed to hydrogen bonding with H2O molecules in as compared to the other alkyl group. Therefore, cyclohexanol is more soluble in water than hexan-1-ol.
Q.62 How will you distinguish between CH3(CH2)3OH and CH3CH=CHCH2OH by a chemical test?
Answer: Distinction can be made by bromine water. CH3CH=CHCH2OH (unsaturated alcohol) decolourises bromine water while CH3(CH2)3OH does not.
Q.63 Why cannot anhydrous calcium chloride be used for drying ethyl alcohol?
Answer: Anhydrous calcium chloride cannot be used for drying ethyl alcohol because it forms an addition compound with the alcohol.
4C2H5OH+CaCl2(anhydrous)→CaCl2.4C2H5OH
Q.64 What happens when propene is reacted with diborane and the product is hydrolysed with alkaline H2O2?
Answer: Propan-1-ol is formed as a result of hydroboration oxidation reaction.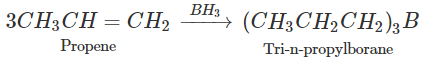 5OH+CaCl2(anhydrous)→CaCl2.4C2H
5OH+CaCl2(anhydrous)→CaCl2.4C2H
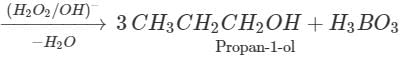
Q.65 What happens when propan-1-ol is treated with ethanoic acid in the presence of concentrated H2SO4?
Answer: Propylethanoate is formed which is an ester.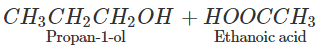
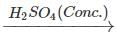
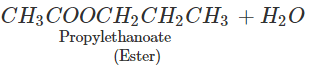
Q.66 What happens when phenol is reacted with excess of bromine water?
Answer: A white precipitate 2, 4, 6-tribromophenol is formed.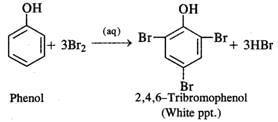
Q.67 How will you distinguish between allyl alcohol and n-propyl alcohol?
Answer: Allyl alcohol will give white turbidity with Lucas reagent immediately, but n-propyl alcohol does not react at room temperature. Actually in allyl alcohol, the allyl carbocation left after the release of OH− ion is resonance stabilized which means that C?OH bond can be very easily cleaved by HCl. But the C?OH bond cleavage is not so easy in n-propyl alcohol because n-propyl carbocation is not resonance stabilised.
CH2=CH−CH2−OH  =CH−CH2−O+H2
=CH−CH2−O+H2 [CH2=CH−
[CH2=CH−
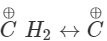 H2
H2
Q.68 Give the product of reaction of ethyl alcohol with conc.H2SO4 at (a) 0°C (b) room temperature (c) 130°C (d) 180ºC.
Answer:
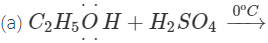
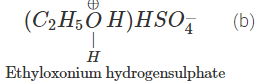
C2H5OH+H2SO4 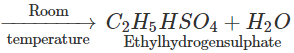
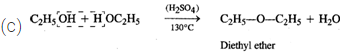
(d) 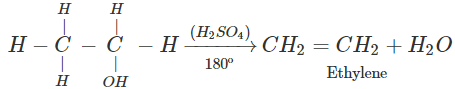
Q.69 Give a simple chemical test to distinguish between: (i) Ethanol and dimethylether (ii) Pentan-1-ol and Pent-1-ene (iii) p-methylphenol and methoxybenzene.
Answer: (i) Ethanol gives iodoform test (yellow ppt.) but dimethylether does not. (ii) Pent-1-ene decolourises bromine water butpentan-1-ol does not. (iii) p-methylphenol gives characteristic violet colouration with FeCl3 but methoxybenzene does not.
Q.70 Why is acid catalysed dehydration of tertiary butyl alcohol faster than that of n-butyl alcohol?
Answer: Acidic dehydration of alcohol involves the formation of carbocation intermediate in a slow step. Since tertiary butylalcohol forms a tertiary butyl carbocation which is more stable than the n-butyl carbocation formed in case of n-butyl alcohol therefore, tertiary butyl alcohol is more reactive than n-butyl alcohol.
Q.71 Unlike phenols, alcohols can be easily protonated. Explain
Answer: In phenols, the lone electron pairs on the oxygen atom are involved in conjugation with the pi electrons of the ring. Hence these are delocalised and are not easily available for protonation. On the other hand, in alcohols the electron pairs on the oxygen atom are not involved in any conjugation. These are localised and are readily available for protonation.
Q.72 How will you distinguish between methanol and ethanol by colour test?
Answer: Ethanol gives a yellow precipitate of iodoform on warming with iodine and sodium carbonate while methanol fails to react.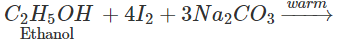
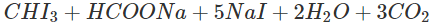
Q.73 What is the function of anhydrous ZnCl2 in the Lucas test of alcohols?
Answer:
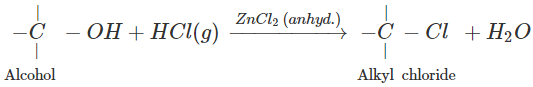
Anhydrous ZnCl2 is a dehydrating agent and absorbs molecules of H2O formed in the reaction. This enables the reaction to proceed in the forward direction.
Q.74 Arrange following in decreasing order of boiling points and account for the order. Pentan-1-ol (A) 2-Methylbutan-2-ol (B) 3-Methylbutan-2-ol (C)
Answer: 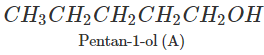
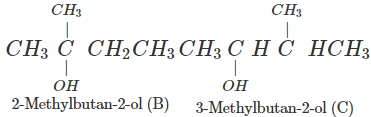
Correct order: A > C > B The boiling points are linked to Van der Waals forces of attraction which depend upon the surface area. Smaller the molecular size, lesser is the surface area or Van der Waals forces and also the boiling point.
Q.75 Predict in which of the following cases, the reaction with Lucas reagent will be immediate, slow or will not take place at all at room temperature?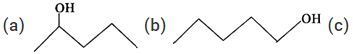
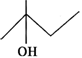
Answer: (a) Structural formula represents a secondary alcohol. Therefore, it will give white turbidity with Lucas reagent after some time i.e. the reaction is slow. (b) Structural formula is of a primary alcohol which gives white turbidity with Lucas reagent only upon heating. (c) Structural formula is of a tertiary alcohol and it will give white turbidity with Lucas reagent immediately.
Q.76 Outline the synthesis of the following alcohols from the indicated starting material (a) Isopropyl alcohol from propane(b) n-Butyl alcohol from ethyne.
Answer:
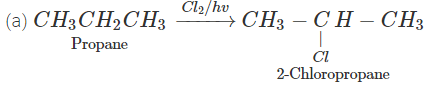
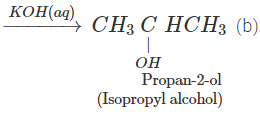
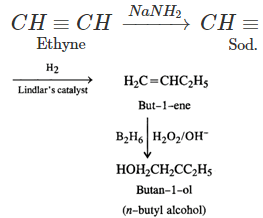
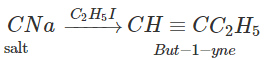
Q.77 Compare the relative acidic strengths of the following : (i) CH3OH (ii)CH3CH2OH (iii)CH3CH(OH)CH3 (iv)(CH3)3COH.
Answer: The relative acidic strengths of the alcohols in an increasing order is :
(CH3)3COH<CH3CH(OH)CH3<CH3CH2OH<CH3OH. The acidic strength of the alcohol is due to the cleavage of OH bond. Thus, greater the number of alkyl groups present or more the size of such groups, difficult will be the bond cleavage and, thus, lesser will be the acidic strength.
Q.78 What is the difference in the nature of alcohol when propene is subjected to acidic hydration or hydroboration oxidation?
Answer: Acidic hydration of propene will give propan-2-ol according to Markovnikov's rule while hydroboration oxidation will yield propan-1-ol according to Anti Markovnikov's rule. For details, consult section 12.6 (Industrial preparation of alkanols).
Q.79 Give the IUPAC names for each of the following compounds.
(a) 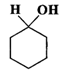 (b)
(b) 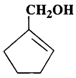 (c)
(c) 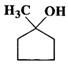 (d)
(d)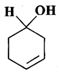
Answer: (a) Cyclohexanol (b) (1-Cyclopentenyl) methanol (c) 1-Methylcyclopentanol (d) 3-Cyclohexen-l-ol
Q.80 Arrange the following compounds in the increasing order of the property indicated against each. Give reasons for your answer. (i) CH3CH2OH,CF3CH2OH,CCl3CH2OH-Acid strength (ii) 2-methyl-2-propanol, 1-butanol and 2-butanol?Reactivity towards sodium.
Answer: (i) Due to -I-effect of the halogen, the electron density in the O-H bond decreases. As a result of this electron-deficiency, the O -H bond weakens and thus facilitates the release of a proton as compared to CH3CH2OH. Further, since F has stronger -I- effect than Cl, therefore, CF3CH2OH is a stronger acid than CCl3CH2OH while CH3CH2OH is the weakest acid. Thus, acid-strength increases in the order : CH3CH2OH<CCl3CH2OH<CF3CH2OH.
(ii) It is an acid-base reaction since alcohols are acidic in nature and sodium is a strong base. As such, the activity of these alcohols towards sodium increases as the acidic character of alcohols increases. Now since the acidic character of alcohols increases in the order: 3° < 2° < 1°, therefore, the reactivity of Na towards alcohols increases in the same order, i.e.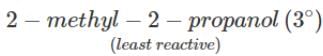 <
< 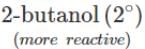 <
< 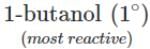
Q.81 Unlike phenols, alcohols are easily protonated or Alcohols are easily protonated in comparison to phenols or Why do phenols not give protonation reactions readily?
Answer: In phenols, the lone pairs of electrons on the oxygen atom are delocalised over the benzene ring due to resonance and hence are not easily available for protonation. In contrast in alcohols, the lone pairs of electrons on the oxygen atom are localized due to absence of resonance and hence are easily available for protonation.
Q.82 How do you account for the fact that unlike phenol, 2, 4-dinitrophenol and 2, 4, 6-trinitrophenol are soluble in aqueous sodium carbonate solution?
Answer: Due to the strong electron-withdrawing nature of −NO2 groups, both 2, 4-dinitrophenol and 2, 4, 6-trinitrophenol are more acidic than carbonic acid and hence dissolve in aq. Na2CO3 solution to form the corresponding sodium salts with the evolution of CO2.
Q.83 Alcohols react with halogen acids or phosphorus halides to form haloalkanes but phenols do not form halobenzenes. Explain.
Answer: The C−O bond in phenols has some double bond character due to resonance and hence cannot be easily cleaved by X− ions in presence of halogen acids or phosphorus halides to form halobenzenes. In contrast, the C−O bond in alcohols is a pure single bond and hence can be easily cleaved by X− ions in presence of halogen acids or phosphorus halides to form haloalkanes.
Q.84 Arrange the following alcohols in order of increasing reactivity towards Lucas reagent : 2-butanol, 1-butanol, 2-methyl-2-propanol
Answer: The order of reactivity of alcohols towards Lucas reagent follows the sequence : 3∘>2∘>1∘. Thus, the correct sequence of reactivity is : 2-methyl-2-propanol(3∘) > 2-butanol (2∘) > 1- butanol (1∘)
Q.85 How will you distinguish between 1-phenylethanol and 2- phenylethanol?
Answer: By iodoform test. 1-Phenylethanol contains the grouping −CHOH−CH3 linked to carbon and hence gives iodoform test while 2- phenylethanol does not contain the grouping −CHOH−CH3 and hence does not give iodoform test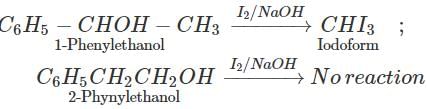
Q.86 Which is a stronger acid, phenol or cresol? Explain.
Answer: Cresol is methyl phenol. It has three positional isomers : o-cresol, m-cresol and p-cresol. Due to +I-effect of CH3 group, electron-density in the O−H bond increases. Therefore, it becomes difficult to break the O−H bond as compared to that in phenol and hence all cresols are less acidic than phenol or phenol is a stronger acid than cresols.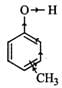 (Due to +I-effect of CH3 group, electron density in the O−H bond increases thereby making the cleavage of O−H bond difficult)
(Due to +I-effect of CH3 group, electron density in the O−H bond increases thereby making the cleavage of O−H bond difficult)
Q.87.Name the reagents which are used in following conversions : (i) A primary alcohol to an aldehyde (ii) Butan-2-one to butan-2-ol (iii) Phenol to 2, 4, 6-tribromophenol.
Answer: Pyridinium chlorochromate (PCC) converts 1∘ alcohols to aldehydes without any danger of being oxidized further to carboxylic acids.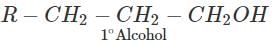
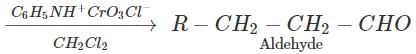
(ii) Butan-2-one can be reduced to butan-2-ol by a variety of reagents such as Ni/H2,Na, alcohol, NaBH4 or LiAlH4.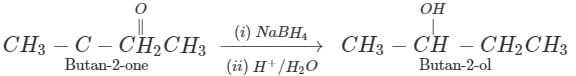
(iii) Phenol reacts with Br2−H2O to give 2, 4, 6-tribromophenol. For structure, refer to page 11/37.
Q.88 Name the reagents and write the chemical equations for the preparation of the following compounds by Williamson synthesis. (i) Ethoxybenzene, (ii) 2-Methyl-2-methoxypropane
Answer: (i) The reagents are sodium phenoxide and bromoethane.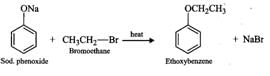
(ii) The reagents are sod. 2-methyl-2-propoxide and bromomethane. 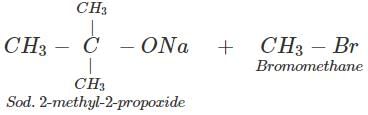
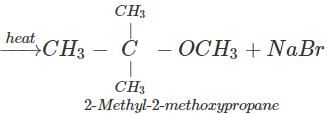
Q.89 Identify the organic product obtained in the following reaction. 2, 3-Dimethylbutan-2-ol 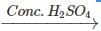
Answer: Dehydration of 2, 3-dimethylbutan-2-ol occurs according to Saytzeff rule as a result of which more highly substituted alkene, i.e., 2, 3-dimethylbut-2-ene is formed as the major product.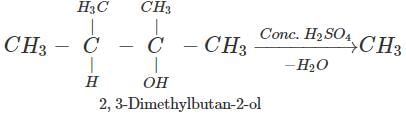
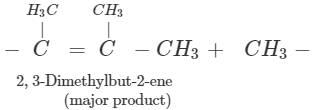
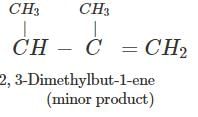
Q.90 Why (CH3)3COH is less acidic than (CH3)3SiOH although carbon is more electronegative than Si?
Answer: Carbon does not have d-orbitals and hence does not form dπ-pπ bonds. In contrast, silicon has empty d-orbitals and hence it forms dπ -pπ bonds with oxygen. As a result, transference of electrons occurs fromO to Si. In other words, electron-density around O decreases. It, therefore, withdraws electrons of the O−H bond towards it. Consequently, O−H bond in (CH3)3SiOH breaks more easily than in (CH3)3COH and hence (CH3)3SiOH is more acidic than(CH3)3COH.
|
75 videos|278 docs|78 tests
|
FAQs on Morrison & Boyd Test: Alcohols, Phenols & Ethers - Chemistry Class 12 - NEET
| 1. What is the Morrison & Boyd Test? |  |
| 2. How is the Morrison & Boyd Test performed? |  |
| 3. What are the applications of the Morrison & Boyd Test? |  |
| 4. Can the Morrison & Boyd Test be used for all alcohols, phenols, and ethers? |  |
| 5. What are the limitations of the Morrison & Boyd Test? |  |

















