Short & Long Answer Questions: Solutions | Physical Chemistry for NEET PDF Download
Q1. Define
(i) Solute
(ii) Solvent
Ans.
(i) Solute - A substance which dissolves in another substance. Its state changes or it is present in smaller quantity.
(ii) Solvent - A component of a solution that has the same physical state as a solution or which is present in larger quantities.
Q2. Is smoke a homogeneous solution?
Ans. No, smoke is a colloidal solution
Q3. Oil and water don't mix. Why?
Ans. Oil is a non-polar ester and water is a polar molecule.

Hence, they do not mix according to like dissolves in like principle.
Q4. Benzene is soluble in toluene but not in water. Why?
Ans. Benzene is a non-polar molecule. Hence it is soluble in toluene which is also non-polar but it is insoluble in water which is polar.
Q5. Which concentration term remains unaffected by temperature?
Ans. Molality remains unaffected by temperature.
Q6. Why does the molality of solution remain unchanged with temperature?
Ans. Because the mass of solute and solvent are independent of temperature.
Q7. Why is molality preferred over the molarity of the solution?
Ans. Because molality does not change with temperature.
Q8. How does the molarity of solution change with temperature?
Ans. With rising in temperature molarity decreases because the volume of the solution increases.
Q9. Explain the term "mole fraction".
Ans. Mole fraction is the ratio of number of moles of solute or solvent and total number of moles of solution.
Q10. Why is vapour pressure of solution of glucose in water lower than that of pure water?
Ans. When glucose is dissolved in water, some of the glucose molecules displace water molecules from the surface. Therefore, the rate of evaporation of water decreases, hence vapour pressure decreases.
Q11. Name two factors on which the vapour pressure of the liquid depends.
Ans. Nature of liquid (intermolecular forces).
Temperature.
Q12. State any two characteristics of ideal solutions.
Ans. They obey Raoult's law for entire range of composition.
There is no change in volume and enthalpy of mixing
Q13. Give an example of ideal solution.
Ans. Solution of benzene and toluene.
Q14. Give an example of a solution showing negative deviation from ideal behaviour.
Ans. Water and sulphuric acid solution show negative deviation from ideal behaviour.
Q15. Give an example of a solution showing positive deviation from ideal behaviour.
Ans. A solution of ethanol in water shows a positive deviation from ideal behaviour.
Q16. What type of solution is formed when chloroform is mixed with acetone?
Ans. When chloroform is mixed with acetone, it shows a negative deviation from ideal behaviour.
Q17. What type of solution is formed when ethanol is mixed with water?
Ans. When ethanol is mixed with water, the solution shows a positive deviation from ideal behaviour.
Q18. Give an example of solution which shows negative deviation from ideality.
Ans. Chloroform Acetone.
Q19. Give an example of a solution which shows positive deviation from ideality.
Ans. Ethanol + Water.
Q20. Define azeotropic mixture.
Ans. A mixture of liquids which boils at constant temperature without a change in composition is called an azeotropic mixture
Q21. What are minimum boiling azeotropes? Give an example.
Ans. A mixture of liquids which boils at a temperature lower than the boiling of both the components in pure state, e.g. Ethanol and water.
Q22. What type of behaviour is expected when water is added to sulphuric acid?
Ans. Water + sulphuric acid shows negative deviation from ideal behaviour.
Q23. What is antifreeze?
Ans. Antifreeze is a liquid which is added to another liquid prevent its freezing at normal freezing point, e.g. Glycol in water.
Q24. Define "colligative properties".
Ans. Those properties which depend upon number of solute particles only and not on nature of solute are called colligative properties.
Q25. Why does the boiling point of a solution increase on adding non-volatile solute?
Ans. On adding non-volatile solute to a liquid its vapour pressure decreases. Hence boiling point increases.
Q26. Why does the freezing of a solution decrease on adding non-volatile solute?
Ans. On adding non-volatile solute to a liquid its vapour pressure decreases, hence the freezing point decreases.
Q27. On what factor does the elevation in boiling point depend?
Ans. The number of solute particles.
Q28. On what factors does depression in freezing point depend?'.
Ans. Nature of liquid (intermolecular force)
Q29. How does the boiling point of a liquid change with external pressure?
Ans. The boiling point of the liquid increases with increasing external pressure.
Q30. What change is observed in the boiling point of a liquid at a higher altitude?
Ans. At a higher altitude boiling point of liquid decreases.
Q31. What is osmosis?
Ans. The process of flow of solvent molecules through a semipermeable membrane from a dilute solution to a concentrated solution.
Q32. Define osmotic pressure.
Ans. The driving force responsible for the process of osmosis is called osmotic pressure. or the extra pressure applied on the solution side to just stop the process of osmosis, when the solvent and solution are separated by semipermeable membrane is called osmotic pressure.
Q33. State how does osmotic pressure vary with temperature?
Ans. Osmotic pressure increases with increasing temperature.
Q34. How does osmotic pressure depend upon number of moles of solute particles?
Ans. Osmotic pressure is directly proportional to the number of solute particles. at constant 'V" and 'T'.
Q35. Doctors advise gargles by saline water in case of sore throat Why?
Ans. Saline water is a hypertonic solution therefore fluids responsible for irritation in throat will come out.
Q36. What is reverse osmosis?
Ans. The flow of solvent from solution side to solvent side separated by the semi-permeable membrane is called reverse osmosis. It occurs when external pressure higher than the osmotic pressure is applied on the solution side.
Q37. What is the use of reverse osmosis?
Ans. Reverse osmosis is used for the desalination of saline water.
Q38. What are isotonic solutions?
Ans. Solutions having the same osmotic pressure are called isotonic solutions.
Q39. Define
(i) Hypertonic Solution
(ii) Hypotonic
Ans. Solution having higher osmotic pressure is called hypertonic solution and that having lower osmotic pressure is called hypotonic solution.
Q40. What is Van't Hoff's factor?
Ans. The ratio of observed colligative properties and normal colligative properties is called Van't Hoff factor. It is denoted by (i).
Q41. When is the value of Van't Hoff factor more than one?
Ans. When solute undergoes dissociation in solution e.g. electrolytes like NaCI.
Q42. When is the value of Van't Hoff factor less than one?
Ans. When the solute molecules undergo association in solution Example: Acetic acid in benzene.
Q43. Name the colligative property mostly used for the determination of molecular mass of macromolecules.
Ans. Osmotic pressure.
Q44. Which will have a greater boiling point
(i) 1 M urea solution
(ii) 1 M NaCI solution
Ans. 1 (M) NaCI solution.
Q45. Osmotic pressure of 1 M KCl solution is higher than 1 M urea solution. Why?
Ans. KCl is an electrolyte, it undergoes dissociation in solution, hence number of particles increases. Urea is non-electrolyte.
Q46. Differentiate between molarity and molality of solution.
Ans. Molarity is the number of moles of solute dissolved in one litre solution. Molality is the number of moles of solute dissolved in one-kilogram solvent.
Molarity decreases with rise in temperature but molality is independent of temperature.
Q47. Give an example each of miscible liquid pairs showing positive and negative deviations from Raoult's law. Give reasons for each such deviation.
Ans. Mixture showing positive deviation from Raoult's law Ethanol and acetone.
Reason: Solute - solvent interaction is weaker than solute - solute and solvent - solvent interactions.
Mixture showing negative deviation from Raoult's law - chloroform and acetone.
Reason: Solute solvent interaction is stronger than solute - solute and solvent - solvent interaction.
Q48. Define vapour pressure. What happens to the vapour pressure when
(i) volatile solute dissolves in the liquid
(ii) non-volatile solute dissolves in the liquid.
Ans. The pressure exerted by gas molecules on its liquid layer at equilibrium is called vapour pressure.
(i) When volatile liquid is added to another liquid then the resultant vapour pressure may increase, decrease or may remain the same depending on the strength of solute-solvent interaction.
(ii) Net vapour pressure decreases when non-volatile solute is added to it.
Q49. Explain why freezing point of a solvent is lowered on dissolving a non-volatile solute in it. Give an important application of freezing point depression.
Ans. When a non-volatile solute is dissolved in a solvent its vapour pressure' decreases. Hence, the vapour pressure of solvent and solution becomes equal at a lower temperature. Application of freezing point depression - To prepare antifreeze mixture used in radiators of vehicles.
Q50. Why dried fruits and vegetables slowly swell when placed in water? What will be the effect of temperature in this process?
Ans. When dried fruits and vegetables are placed in water they swell due to flow of water into them by osmosis. Increasing temperature speeds up the process.
Q.51. Define osmosis and osmotic pressure.
Ans. Osmosis: The process of flow of solvent from a dilute solution to a concentrated solution through a semipermeable membrane is called osmosis.
Osmotic pressure: The driving force responsible for the process of osmosis is called osmotic pressure.
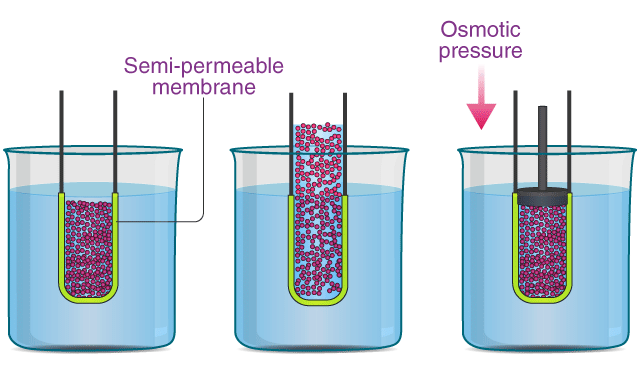 Q.52. What is meant by abnormal molecular mass of solute? Which factor is responsible for abnormality in molecular masses of solutes using colligative properties?
Q.52. What is meant by abnormal molecular mass of solute? Which factor is responsible for abnormality in molecular masses of solutes using colligative properties?
Ans. When the observed value of molecular mass is different from the normal value, it is called abnormal molecular mass. Abnormality in molecular mass arises when solute undergoes association or dissociation in the solution.
Q.53. The molecular mass of ethanoic acid when determined by colligative property of its solution is abnormal. Explain why?
Ans. Ethanoic acid undergoes association in non-polar solvent and dissociation in polar solvent. Hence, its molecular mass is abnormal.
Q.54.
(a) What is solubility of a substance?
(b) On what factors solubility of solid depends?
(c) What is the effect of pressure on solubility?
Ans. Refer text 2.1.2 and 2.1.3.
Q.55.
(a) Define vapour pressure.
(b) State and explain Raoult's law for a solution of volatile liquids.
(c) State Raoult's law for a solution of non-volatile solute.
Ans.
(a) Refer text 2.3.
(b) Refer text 2.3.1.
(c) Refer text 2.3.4.
Q.56. What are ideal and non-ideal solutions? Explain each with diagram and suitable examples.
Ans. Refer text 2.3.2.
Q.57. Explain the terms:
(a) Elevation in boiling point.
(b) Depression in freezing point.
(c) Relative lowering of vapour pressure.
(d) Osmotic pressure.
Ans.
(a) Refer text 2.4.2.
(b) Refer text 2.4.3.
(c) Refer text 2.4.1.
(d) Refer test 2.4.4.
Q.58. What is van't -Hoff factor? How is it used to calculate molecular mass of a non-volatile substances?
Ans. Refer test 2.6.
Q.59. What happens when RBC are placed in
(i) 1% NaCI solution.
(ii) Pure water.
Ans.
(i) RBC is hypotonic with 1% NaCI hence it will shrink due to plasmolysis.
(ii) RBC is hypertonic with pure water hence it will swell or even burst due to hemolysis.
Q.60. 4% NaOH solution (mass/volume) and 6% urea solution (mass/volume) are equimolar but not isotonic. Why?
Ans. Both the solutions 4% NaOH (WIV) and 6% urea (WIV) have same concentration (1 M) but these are not isotonic because NaOH undergoes dissociation in solution. Therefore, number of particles in NaOH solution is more than that in urea solution.
Q.61. Name the solid that is separated when salt solution is slowly frozen.
Ans. Ice separates out first because freezing point of salt solution is less than pure water.
Q.62. Why do aquatic species feel more comfortable in winter than in summer?
Ans. In winter season at low temperature solubility of oxygen in water is higher than that in summer at high temperature. Hence, species feel more comfortable in winter than in summer.
Q.63. What precaution should be taken in intravenous injection?
Ans. The medicine to be given by intravenous injection should be isotonic with blood plasma. If hypertonic solution is injected intravenously then RBC will shrink (crenation). If hypotonic solution is injected intravenously then RBC will swell or even burst (hemolytic).
Q.64. A mixture of ethyl alcohol and water cannot be separated into pure component by fractional distillation. Why?
Ans. Ethyl alcohol and water form an azeotropic mixture which distills over as a pure liquid and cannot be separated by fractional distillation.
Q.65. Give one example of interstitial and substitutional solid solutions.
Ans. Tungsten carbide is an example of interstitial solid solution while brass (alloy of Cu and Zn) represents substitutional solid solution.
Q.66. How is Henry's Law constant related to the solubility of a gas in a solvent?
Ans. KH is inversely proportional to the solubility of a gas in a solvent.
Q.67. How can you compare the relative solubilities of different salts in the same solvent?
Ans. The relative solubilities can be compared in terms of solubility product constant (Ksp) values i.e., more the Ksp value, more will be the solubility of the salt.
Q.68. What does normality (N) represent?
Ans. It represents the number of gram equivalents of the solute dissolved per litre of the solution. Its unit is equivalent. L-1.
Q.69. Why does not molality of the solution change with temperature?
Ans. It takes into account only the mass of the solute as well of the solvent. Both of them do not change with change in temperature.
Q.70. What are the units of mole fraction?
Ans. Mole fraction being a ratio has no units.
Q.71. What should be the nature of the solution while representing the solubility of a solute?
Ans. Solution must be saturated in nature.
Q.72. Rubbing isopropyl alcohol often gives a cooling sensation to the skin. Why?
Ans. Isopropyl alcohol (CH3CHOHCH3) being a volatile liquid absorbs the required latent heat of vaporization from the skin thereby giving a cooling sensation.
Q.73. What will happen to the boiling point of the solution on mixing two miscible liquids showing negative deviation from Raoult's law?
Ans. The vapour pressure of the solution will decrease and its boiling point will increase.
Q.74. The bottle of liquid ammonia is generally cooled before opening the seal. Assign reason.
Ans. On cooling, the gas will tend to liquefy and its vapour pressure will decrease. Therefore, the gas will not come with force upon opening the seal.
Q.75. Does osmosis occur from hypertonic solution to hypotonic solution?
Ans. No, it always occurs from hypotonic to hypertonic solution.
Q.76. What type of azeotrope will result on mixing chloroform and acetone?
Ans. It will result in maximum boiling azeotrope because liquid mixture shows negative deviation from Raoult's Law.
Q.77. Name the most commonly used semi permeable membrane in the laboratory.
Ans. It is copper ferrocyanide Cu2[Fe(CN)6] and is formed by mixing equimolar aqueous CuSO4 and K4[Fe(CN)6] solutions
Q.78. The Van't Hoff factor of a solution is 1. What does it indicate?
Ans. It indicates that the solute does not undergo either dissociation or association in solution.
Q.79. What is the purpose of adding ethylene glycol to water?
Ans. Ethylene glycol (CH2OHCH2OH) is added to water to lower its freezing point. It is known as antifreeze solution.
Q.80. Why does the solubility of NaCl in water increase with the rise in temperature?
Ans. Because the process of dissolution is of endothermic nature.
Q.81. What is the effect of temperature on the molarity of a solution?
Ans. It changes with the change in temperature.
Q.82. Can a solution of its own have osmotic pressure?
Ans. No, it can have only vapour pressure and not osmotic pressure.
Q.83. Two liquids A and B upon mixing form a warm solution. What type of deviations do they show from Raoult's Law?
Ans. Since energy is released in the form of heat when the two liquids A and B are mixed, they show negative deviation from Raoult's Law.
Q.84. What will happen when red blood corpuscles (RBCs) are placed in
(a) 1 % NaCI solution
(b) 0.6% NaCI solution?
Ans. We all know that RBCs are isotonic with 0.9% NaCI solution.
(a) If RBCs are placed in contact with 1 % NaCI solution, then the osmotic pressure of 1 % NaCI will be higher than that RBCs. As a result, water present inside the cell moves into the NaCI solution through cell walls acting as semi-permeable membrane. The RBCs will therefore, shrink.
(b) However, reverse will take place in case these are kept in contact with 0.6% NaCI solution which has less osmotic pressure. Water will now move into the RBCs and they will swell.
Q.85. What should be the maximum concentration of the solute in case a solution is to be ideal?
Ans. It should not be more than 5 % either W/W or W/V.
Q.86. What will the nature of the solid solution formed on mixing two solids with large difference in particle size?
Ans. The solution is known as interstitial solid solution.
Q.87. Which colligative property is generally used for determining the molar mass of a solute?
Ans. Osmotic pressure is generally used for this purpose.
Q.88. What is common in all the four colligative properties?
Ans. All of them depend upon the number of the particles of the solute in the solution as well as its molar concentration.
Q.89. Will osmosis take place when 0.1 M aqueous urea and glucose solutions are separated by semi permeable membrane?
Ans. No, it will not take place as they are isotonic as well as iso-osmotic in nature.
Q.90. The cryoscopic constant (Kf) for water is 1.86 K mol−1kg−1. What does it signify?
Ans. It signifies that when 1 mole of a normal solute is dissolved in one kg of water, the freezing point of water is lowered by 1.86 K.
Q.91. How does osmotic pressure depend upon temperature?
Ans. It increases with the rise in temperature (π = CRT).
Q.92. Which aqueous solution has higher concentration; 1 molar or 1 molal having the same solute?
Ans. In aqueous solution, the density of water is normally taken as one. This means that 1m solution has 1 mole of the solute dissolved in 1000 g or 1000 mL of water. At the same time, 1M solution contains 1 mole of the solute in 1000mL solution which is the volume of both the solute and the solvent present in the solution. This clearly shows that the solvent present in 1M solution is less as compared to 1 m solution. Therefore, 1M solution is more concentrated than 1 m solution.
Q.93. Why is molality of a solution preferred for expressing concentration over molarity?
Ans. In molality, the mass of the solvent is considered while in expressing molarity, volume of solution is taken into account. Since mass does not change with temperature while volume changes, molality does not change with temperature but molarity changes. Hence, molality is better for expressing the concentration of solution than molarity.
Q.94. Will elevation in boiling point temperature be same for 0⋅1 MNaCI and 0⋅1M sucrose solution?
Ans. No it will not be the same because colligative property does not depend upon molar concentration alone but also upon the number of particles (ions) in solution. Sodium chloride (NaCI) is an ionic solid and will form more number of particles (ions)in solution than sucrose (C12H22O11) which is a molecular solid. Thus, the elevation in boiling point for 0.1 M NaCl solution will be more.
Q.95. What is the expected deviation from ideal solution behaviour when acetone and chloroform are mixed to form a solution?
Ans. The molecules of acetone and chloroform will get linked by intermolecular hydrogen bonding. This means that the volume of the solution will be less than for ideal solution. Therefore, ΔV(mix) will be negative and solution will show negative deviation as compared to ideal solution.
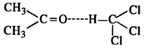
|
117 videos|226 docs|237 tests
|
FAQs on Short & Long Answer Questions: Solutions - Physical Chemistry for NEET
| 1. What are solutions? |  |
| 2. How can solutions be classified? |  |
| 3. What is the difference between a solute and a solvent? |  |
| 4. How can the concentration of a solution be measured? |  |
| 5. What are some common examples of solutions in everyday life? |  |





















