Carbohydrates: Monosaccharides, Disaccharides & Polysaccharides | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Carbohydrates |

|
| Classification and structure of Carbohydrates |

|
| 1. Monosaccharides |

|
| 2. Polysaccharides: Starch, Cellulose & Glycogen |

|
Carbohydrates
Carbohydrates are one of the three main macronutrients, along with proteins and fats, that provide energy for the body. They are organic compounds made up of carbon, hydrogen, and oxygen atoms.
Carbohydrates are polyhydroxy aldehydes and ketones and substances which hydrolyse to polyhydroxy aldehydes and ketones. Carbohydrates received their name because of their general formula Cx(H2O)y, according to which they appear to be hydrates of carbon.
xCO2 + yH2O Cx(H2O)y + xO2
Photosynthesis:
6CO2 + 6H2O+ 18 ATP C6H12O6 + 6O2
Cellular Respiration:
C6H12O6 +6O2 6CO2 +6H2O +38 ATP (36 ATP net gain)
Classification and structure of Carbohydrates
The simplest carbohydrates are called sugars or saccharides, (Latin: Saccharum, sugar). Carbohydrates can be classified as monosaccharides, oligosaccharides and polysaccharides.
1. Monosaccharides
(i) General Characteristic of Monosaccharides:
The important characteristics of monosaccharides as follows:
- All monosaccharides are water soluble due to the presence of hydrogen bonding between the different OH groups and surrounding water molecules.
- Monosaccharides have sweet taste and dependent upon heating they get charred and give the smell of burning sugar.
- Monosaccharides are optically active in nature due to the presence of chiral carbon atoms.
- The chemical characteristics of monosaccharides are due to OH groups and carbonyl group which may be either aldehydic or ketonic group.
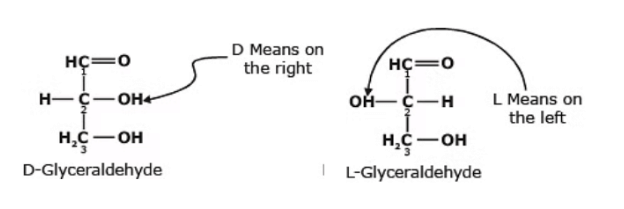
- Glyceraldehyde contains one asymmetric carbon atom (marked by an astrisk) and can thus exist in two optically active forms, called the D-form and the L-form. Clearly, the two forms are mirror images that cannot be superimposed, that is they are enantiomers.
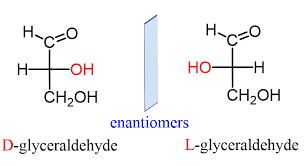
- All four isomers have been prepared synthetically. The D-and L-erythrose are mirror images, that is, they are enantiomers.
- They have exactly the same degree of rotation but in opposite directions. Equal amounts of the two would constitute a racemic mixture, that is a mixture that would allow a plane-polarised light to pass through the solution unchanged.
- Supplying hydrogen atoms to the five carbon atoms to satisfy their tetravalency, following structure (open chain) may be assigned to glucose: (* indicates assymetric carbon atom).
(ii) Structure Formulas for Monosaccharides
- Although many of the properties of D(+)-glucose can be explained in terms of an open-chain structure (1, 2, or 3), a considerable body of evidence indicates that the open-chain structure exists, primarily, in equilibrium with two cyclic forms.
- These can be represented by structures 4 and 5 or 6 and 7. The cyclic forms of D(+)-glucose are hemiacetals formed by an intramolecular reaction of the -OH group at C5 with the aldehyde group.
- Cyclization creates a new stereogenic centre at C1, and this stereogenic centre explains how two cyclic forms are possible. These two cyclic forms are diastereomers that are different only in the configuration of C1. In carbohydrates, chemistry diastereomers of this type are called anomers, and the hemiacetal carbon atom is called the anomeric carbon atom.
Structures 4 and 5 for the glucose anomers are called Haworth formulas and, although they do not give an actual picture of the shape of the six-membered ring, they have many practical uses. It demonstrates how the representation of each stereogenic centre of the open-chain form can be correlated with its representation in the Haworth formula.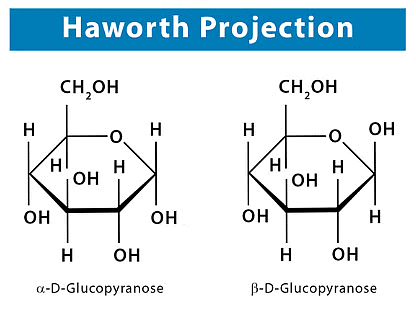
Each glucose anomer is designated as an a anomer or b anomer depending on the location of the -OH group of Cl. When we draw the cyclic forms of a D sugar:
(iii) Mutarotation:
- Ordinary D(+)-glucose has a melting point of 146°C. However, when D(+)-glucose is crystallized by evaporating an aqueous solution kept above 98°C, a second form of D(+)-glucose with a melting point of 150°C can be obtained.
- When the optical rotations of these two forms are measured, they are found to be significantly different, but when an aqueous solution of either form is allowed to stand, its rotation changes. The specific rotation of one form decreases and the rotation of the other increases, until both solutions show the same value.
- A solution of original D-(+)glucose (mp: 146°C) has an initial specific rotation of 112°, but, ultimately, the specific rotation of this solution falls to 52.7°. A solution of second form of D(+) glucose (mp: 150°C) has an initial specific rotation of 18.7°, but slowly, the specific rotation of this solution rises to 52.7°. This change in rotation towards an equilibrium value is called mutarotation.
- The explanation for this mutarotation lies in the existence of an equilibrium between the open-chain form of D(+) glucose and the a and b forms of the cyclic hemiacetals.
(iv) Conversion to Esters:
Treating a monosaccharide with excess acetic anhydride and a weak base (such as pyridine or sodium acetate) converts all of the hydroxyl groups, including the anomeric hydroxyl, to ester groups. If the reaction is carried out at a low temperature (e.g., 0°C), the reaction occurs stereospecifically; the α-anomer gives the a-acetate and the β-anomer gives the b-acetate. Acetate esters are common protecting groups for carbohydrate hydroxyls.
(v) Benedict's Or Tollens' Reagents: Reducing Sugars
- Benedict's reagent (An alkaline solution containing a cupric citrate complex ion) and Tollen's solution [Ag (NH3)2OH] _ oxidize and thus give positive tests with aldoses and ketoses. The tests are positive even though aldoses and ketoses exist primarily as cyclic hemiacetals.
- Sugars that give positive tests with Tollen's or Benedict's solutions are known as reducing sugars, and all carbohydrates that contain a hemiacetal group give positive tests.
- In aqueous solution these hemiacetals exist in equilibrium with relatively small, but not insignificant, concentration of noncyclic aldehydes or α-hydroxy ketones. It is the latter two that undergoes the oxidation until one reactant is exhausted.
- Carbohydrates that contain only acetal groups do not give positive tests with Benedict's or Tollen's solutions, and they are called non-reducing sugars. Acetals do not exist in equilibrium with aldehydes or α-hydroxy ketones in the basic aqueous media of the test reagents.
(vi) Bromine Water : The synthesis of Aldonic Acid
- Monosaccharides do not undergo isomerization and fragmentation reactions in mildly acidic solution. Thus, a useful oxidizing reagent for preparative purposes is bromine in water (pH 6.0).
Bromine water is a general reagent that selectively oxidizes the -CHO group to a -CO2H group. It converts an aldose to an aldonic acid. - Experiments with aldopyranoses have shown that the actual course of the reaction is somewhat more complex than we have indicated above. Bromine water specifically oxidizes the β anomer, and the initial product that forms is a d-aldonolactone.
- This compound may then hydrolyze to an aldonic acid, and the aldonic acid may undergo a subsequent ring closure to form a γ-aldonolactone:
(vii) Nitric Acid Oxidation: Aldaric Acid
Dilute nitric acid -a stronger oxidizing agent than bromine water oxidizes the both -CHO group and the terminal -CH2OH group of an aldose to -CO2H groups.
These dicarboxylic acids are known as aldaric acids:
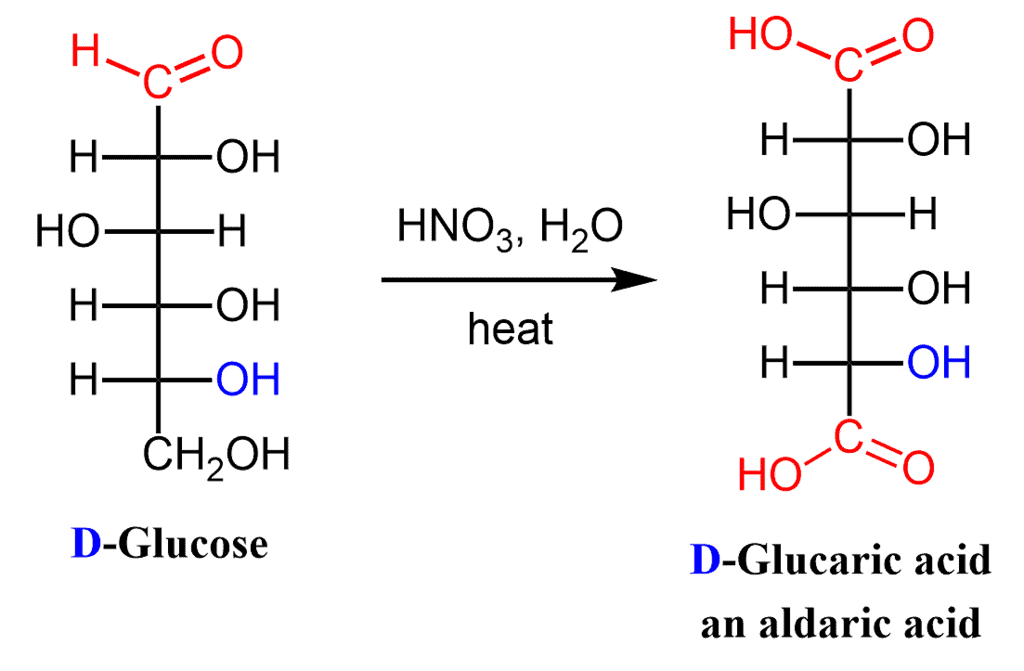
This aldaric acid obtained from D-glucose is called D-glucaric acid.
(viii) Periodate Oxidation: Oxidative Cleavage Of Polyhydroxy Compounds
- Compounds that have hydroxyl groups on adjacent atoms undergo oxidative cleavage when they are treated with aqueous periodic acid (HIO4). The reaction breaks carbon-carbon bonds and produces carbonyl compounds (aldehydes, ketones, or acids).
+ HIO4 → 2
+ HIO3 + H2O
- Since the reaction usually takes place in quantitative yield, valuable information can often be gained by measuring the number of molar equivalents of periodic acid that is consumed in the reaction as well as by identifying the carbonyl products.
Periodate oxidation are though to take place through a cyclic intermediate: - In these periodate oxidation that for every C - C bond broken, a C - O bond is formed at each carbon.
- When three or more -CHOH groups are continuous, the internal ones are obtained as formic acid. Periodate oxidation of glycerol, for example, gives two molar equivalents of formaldehyde and one molar equivalent of formic acid.
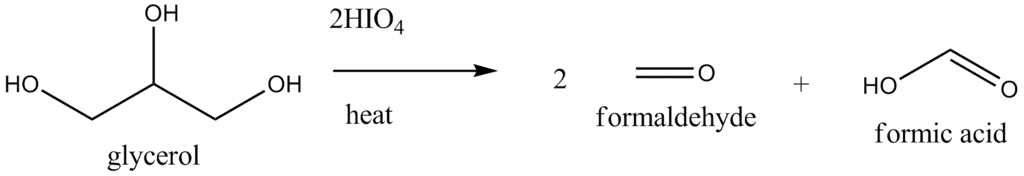
- Oxidative cleavage also take place when an - OH group is adjacent to the carbonyl group of an aldehyde or ketone (but not that of an acid or an ester). Glyceradehyde yields two molar equivalents of formic acid and one molar equivalent of formaldehyde, while dihydroxyacetone gives two molar equivalents of formaldehyde and one molar equivalent of carbon dioxide.
- Periodic acid does not cleave compound in which the hydroxyl groups are separated by an intervening -CH2 group, nor those in which a hydroxyl group is adjacent to an ether or acetal.
(ix) Reduction OF Monosaccharides: Alditols
Aldoses (and ketoses) can be reduced with sodium borohydride to compounds called alditols:
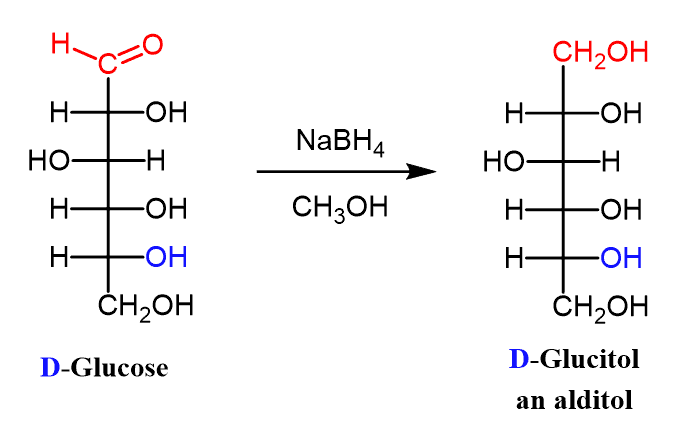
Reduction of D-glucose, for example, yields D-glucitol.
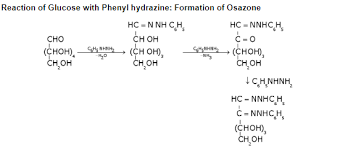
(x) Reactions of Monosaccarides with Phenylhydrazine: Osazones
- The aldehyde group of an aldose reacts with such carbonyl reagents as hydroxylamine and phenylhydrazine.
- With hydroxylamine, the product is the expected oxime. With enough phenylhydrazine, however, three molar equivalents of phenylhydrazine are consumed and a second phenylhydrazeone group is introduced at C2.
- The product is called a phenylosazone. Phenylosazones crystallize readily (unlike sugars) and are useful derivatives for identifying sugars.
- The mechanism for osazone formation probably depends on a series of reaction in which it behaves very much like
in giving a nitrogen version of an enol.
A Mechanism for the Reaction - Phenylosazone Formation
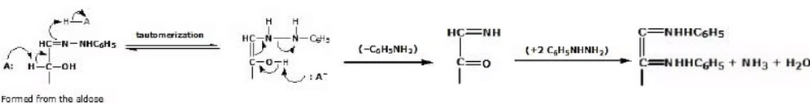
Osazone formation results in a loss of the stereogenic centre at C2 but does not affect other stereogenic carbons; D-glucose and D-mannose, for example, yield the same phenylosazone:
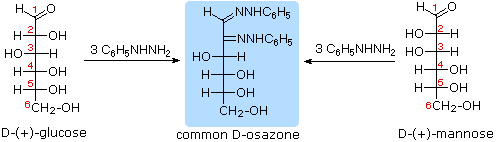
This experiment, first done by Emil Fischer, established that D-glucose and D-mannose have the same configuration about C3, C4 and C5. Diastereomeric aldoses that differ in configuration at only one carbon (such as D-glucose and D-mannose) are called epimers. In general, any pair of diastereomers that differ in configuration at only a single tetrahedral stereogenic carbon can be called as epimers.
Epimers
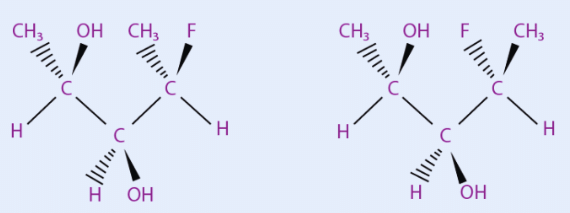
Many common sugars are closely related, differing only by the stereochemistry at a single carbon atom. For example, glucose and mannose differ only at C2, the first asymmetric carbon atom.
Sugars that differs only by the stereochemistry at a single carbon are called epimers and the carbon atom where they differ is generally stated.
 Epimers
Epimers
If the number of a carbon atom is not specified, it is assumed to be C2. Therefore, glucose and mannose are "C2 epimers" or simply "epimers". The C4 epimer of glucose is galactose and the C2 epimer of erythrose is threose.
2. Polysaccharides: Starch, Cellulose & Glycogen
Polysaccharides
Polysaccharides are the polymers of monosaccharides. The natural polysaccharides generally contain about 100-3000 monosaccharide units. The three most abundant natural polysaccharides-cellulose, starch and glycogen are derived from the same monomer, i.e., glucose.
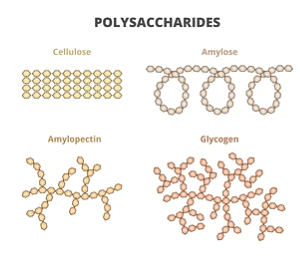
(i) Starch
- It is a polymer of glucose. Its molecular formular is (C6H10O5)n where the value of n = (200 - 1000) varies from source to source.
- It is the chief food reserve material or storage polysaccharide of plants and is found mainly in seeds, roots, tubers, etc. Wheat, rice, potatoes, corn, bananas etc., are rich source of starch.
- Starch is not a single compound but is a mixture of two components - a water soluble component called amylose (20%) and a water insoluble component called amylopectin (80%). Both amylose and amylopectin are polymers of α-D glucose.
- Hydrolysis: Hydrolysis of starch with hot dilute acids or by enzmyes give dextrins of varying complexity, maltose and finally D-glucose. Starch does not reduce Tollen's reagent and Fehling's solution.
- Uses:
- It is used in coating and sizing paper to improve the writing qualities.
- Starch is used to treat textile fibres before they are woven into cloth so that they can be woven without breaking.
- It is used in manufacture of dextrins, glucose and ethyl alcohol.
- Starch is also used in manufacture of starch nitrate, which is used as an explosive.
- It is used as a food. It is encountered daily in the form of potatoes, bread, cakes, rice etc.
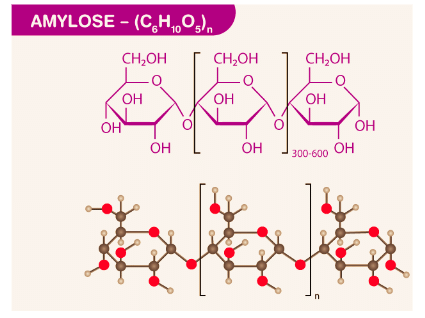
(ii) Amylose
- It is a linear polymer of α-D glucose.
- It contains about 200 glucose units which are linked to one another through a-linkage involving C1 of one glucose unit with C4 of the other.
(iii) Amlopectin
- It is a highly branched polymer.
- It consists of a large number (several branches) of short chains each containing 20-25 glucose units which are joined together through α-linkages involving C1 of one glucose unit with C4 of the other.
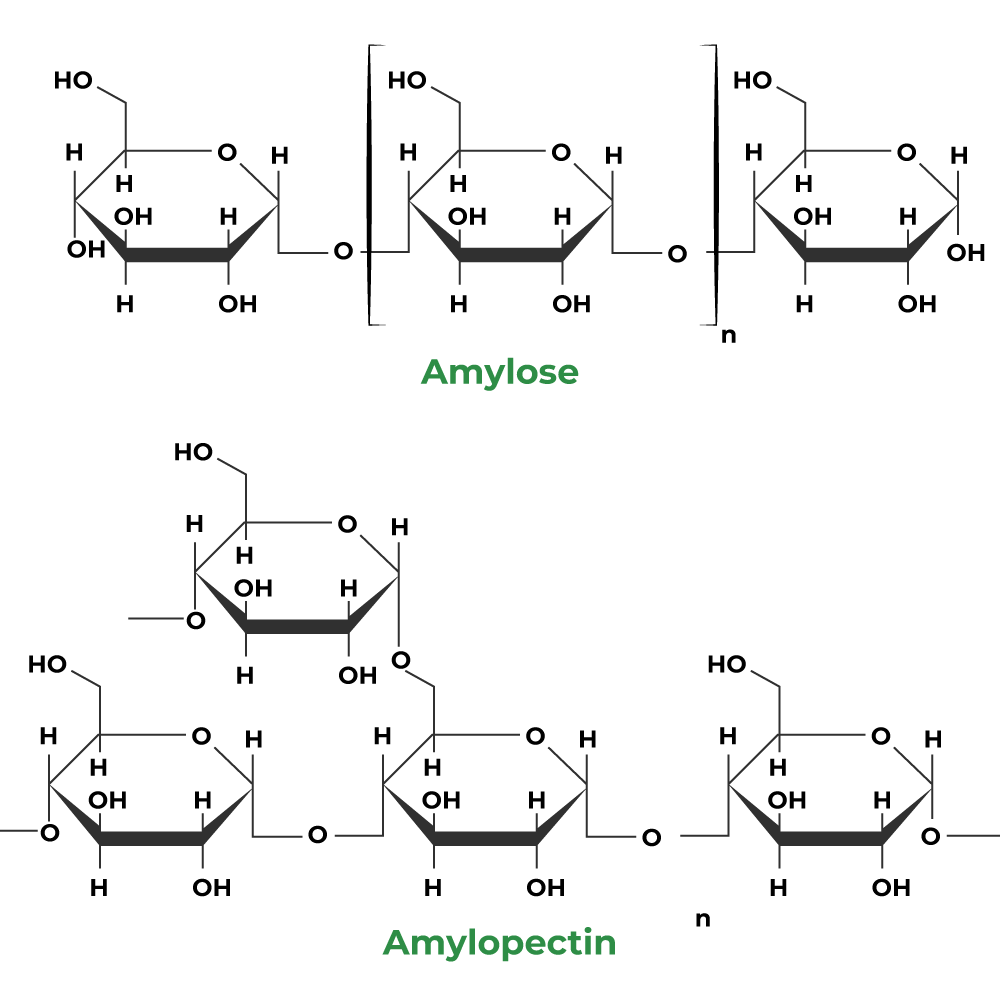
- The C1 of terminal glucose unit in each chain is further linked to C6 of the other glucose unit in the next chain through C1-C6 α-linkage.
- This gives amylopectin a highly branched structure as shown below
(iv) Cellulose
- Cellulose is the chief component of wood and plane fibres; cotton, for instance, is nearly pure cellulose. It is insoluble in water and tasteless; it is a non-reducing carbohydrate. These properties, in part at least, are due to extremely high molecular weight.
- Cellulose has the formula (C6H10O5)n . Complete hydrolysis by acid yields D( )-glucose as the monosaccharide. Hydrolysis of completely methylated cellulose gives a high yield of 2, 3, 6-tri-O-methyl-D-glucose. Like starch, therefore, cellulose is made up of chains of D-glucose units, each unit joined by a glycoside linkage of C-4 of the next.
- Cellulose differs from starch, however, in the configuration of the glycoside linkage. Upon treatment with acetic anhydride and sulphuric acid, cellulose yields octa-O-acetylcholine, there is evidence that all glycoside linkages in cellulose, like the one in ( ) cellobiose, are beta linkages.
- Physical methods give molecular weights for cellulose ranging from 250000 to 1000000 or more; it seems likely that there are at least 1500 glucose units per molecule. End group analysis by both methylation and periodic acid oxidation gives a chain length of 1000 glucose units or more. X-ray analysis and electron microscopy indicate that these long chains lie side by side in bundles, undoubtedly held together by hydrogen bonds between the numerous neighbouring -OH groups. These bundles are twisted together to form.
- Rope like structure which themselves are grouped to form the fibers we can see. In wood, these cellulose "ropes" are embedded in lignin to give a structure that has been linked to reinforced concrete.
Properties of Cellulose:
- We have seen that the glycoside linkages of cellulose are broken by the action of acid, each cellulose molecule yielding many molecules of D(+)-glucose. Now let us look briefly at reactions of cellulose in which the chain remains essentially intact.
- Each glucose unit in cellulose contains three free -OH groups; these are the positions at which reactions occur.
- These reactions of cellulose, carried out to modify the properties of a cheap, available ready-made polymer, are of tremendous industrial importance.
- Like any alcohol, cellulose forms esters. Treatment with a mixture of nitric and sulphuric acid converts cellulose into cellulose nitrate.
- The properties and uses of the product depend upon the extent of nitration. Guncotton, which is used in making smokeless powder, is very nearly completely nitrated cellulose, and is often called cellulose trinitrate (three nitrate groups per glucose unit).
- Pyroxylin is less highly nitrated material containing between two and three nitrate groups per glucose unit. It is used in the manufacture of plastics like celluloid and collodion, in photographic film, and in lacquers. It has the disadvantage of being flammable, and forms highly toxic nitrogen oxides upon burning.
- Industrially, cellulose is alkylated to ethers by the action of alkyl chlorides (cheaper than sulfates) in the presence of alkali.
- Considerable degradation of the long chain is unavoidable in these reactions. Methyl, ethyl, and benzyl others of cellulose are important in the production of textiles, films, and various plastic objects.
|
75 videos|278 docs|78 tests
|
FAQs on Carbohydrates: Monosaccharides, Disaccharides & Polysaccharides - Chemistry Class 12 - NEET
| 1. What are carbohydrates and how are they classified? |  |
| 2. What are monosaccharides and what are their structure formulas? |  |
| 3. What is mutarotation in carbohydrates? |  |
| 4. How can reducing sugars be detected using Benedict's or Tollens' reagents? |  |
| 5. What are some examples of polysaccharides and what are their functions? |  |















